The active components of Erzhi wan and their anti-Alzheimer's disease mechanisms determined by an integrative approach of network pharmacology, bioinformatics, molecular docking, and molecular dynamics simulation
- PMID: 39027618
- PMCID: PMC11255520
- DOI: 10.1016/j.heliyon.2024.e33761
The active components of Erzhi wan and their anti-Alzheimer's disease mechanisms determined by an integrative approach of network pharmacology, bioinformatics, molecular docking, and molecular dynamics simulation
Abstract
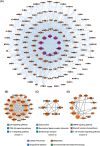
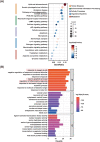
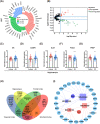
Erzhi Wan (EZW), a classic Traditional Chinese Medicine formula, has shown promise as a potential therapeutic option for Alzheimer's disease (AD), yet its mechanism remains elusive. Herein, we employed an integrative in-silico approach to investigate the active components and their mechanisms against AD. We screened four active components with blood-brain barrier permeabilities from TCMSP, along with 307 corresponding targets predicted by SwissTargetPrediction, PharmMapper, and TCMbank websites. Then, we retrieved 2260 AD-related targets from Genecards, OMIM, and NCBI databases. Furthermore, we constructed the protein-protein interaction (PPI) network of the intersected targets via the STRING database and performed the GO and KEGG enrichment analyses using the "clusterProfiler" R package. The results showed that the intersected targets were intimately related to the p53/PI3K/Akt signaling pathway, serotonergic synapse, and response to oxygen level. Subsequently, 25 core targets were found differentially expressed in brain regions by bioinformatics analyses of GEO datasets of clinical samples from the Alzdata database. The binding sites and stabilities between the active components and the core targets were investigated by the molecular docking approach using Autodock 4.2.6 software, followed by pocket detection and druggability assessment via the DoGSiteScorer server. The results showed that acacetin, β-sitosterol, and 3-O-acetyldammarenediol-II strongly interacted with the druggable pockets of AR, CASP8, POLB, and PREP. Eventually, the docking results were further cross-referenced with the literature research and validated by 100 ns of molecular dynamics simulations using GROMACS software. Binding free energies were calculated via MM/PBSA strategy combined with interaction entropy. The simulation results indicated stable bindings between four docking pairs including acacetin-AR, acacetin-CASP8, β-sitosterol-POLB, and 3-O-acetyldammarenediol-II-PREP. Overall, our study demonstrated a theoretical basis for how three active components of EZW confer efficacy against AD. It provides a promising reference for subsequent research regarding drug discoveries and clinical applications.
Keywords: AD pathology; Computational biology; Erzhi wan; Network pharmacology; Oxidative stress; p53/PI3K/Akt signaling pathway.
© 2024 The Authors.
Conflict of interest statement
None Declared.
Figures





Similar articles
-
Integration of Network Pharmacology and Molecular Docking Technology Reveals the Mechanism of the Therapeutic Effect of Xixin Decoction on Alzheimer's Disease.Comb Chem High Throughput Screen. 2022;25(10):1785-1804. doi: 10.2174/1386207325666220523151119. Comb Chem High Throughput Screen. 2022. PMID: 35616676
-
Exploring Acori Tatarinowii Rhizoma and Polygalae Radix in Alzheimer's: Network pharmacology and molecular docking analysis.Medicine (Baltimore). 2024 Apr 12;103(15):e37740. doi: 10.1097/MD.0000000000037740. Medicine (Baltimore). 2024. PMID: 38608086 Free PMC article.
-
Research on the Regulatory Mechanism of Ginseng on the Tumor Microenvironment of Colorectal Cancer based on Network Pharmacology and Bioinformatics Validation.Curr Comput Aided Drug Des. 2024;20(5):486-500. doi: 10.2174/1573409919666230607103721. Curr Comput Aided Drug Des. 2024. PMID: 37287284
-
Network Pharmacology, Molecular Docking and Experimental Verification Revealing the Mechanism of Fule Cream against Childhood Atopic Dermatitis.Curr Comput Aided Drug Des. 2024;20(6):860-875. doi: 10.2174/0115734099257922230925074407. Curr Comput Aided Drug Des. 2024. PMID: 37807411
-
Exploration of the mechanism of Zisheng Shenqi decoction against gout arthritis using network pharmacology.Comput Biol Chem. 2021 Feb;90:107358. doi: 10.1016/j.compbiolchem.2020.107358. Epub 2020 Aug 8. Comput Biol Chem. 2021. PMID: 33243703 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

