Emerging opportunities to target inflammation: myocardial infarction and type 2 diabetes
- PMID: 39027945
- PMCID: PMC11416061
- DOI: 10.1093/cvr/cvae142
Emerging opportunities to target inflammation: myocardial infarction and type 2 diabetes
Abstract
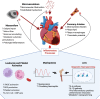
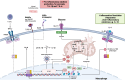
After myocardial infarction (MI), patients with type 2 diabetes have an increased rate of adverse outcomes, compared to patients without. Diabetes confers a 1.5-2-fold increase in early mortality and, importantly, this discrepancy has been consistent over recent decades, despite advances in treatment and overall survival. Certain assumptions have emerged to explain this increased risk, such as differences in infarct size or coronary artery disease severity. Here, we re-evaluate that evidence and show how contemporary analyses using state-of-the-art characterization tools suggest that the received wisdom tells an incomplete story. Simultaneously, epidemiological and mechanistic biological data suggest additional factors relating to processes of diabetes-related inflammation might play a prominent role. Inflammatory processes after MI mediate injury and repair and are thus a potential therapeutic target. Recent studies have shown how diabetes affects immune cell numbers and drives changes in the bone marrow, leading to pro-inflammatory gene expression and functional suppression of healing and repair. Here, we review and re-evaluate the evidence around adverse prognosis in patients with diabetes after MI, with emphasis on how targeting processes of inflammation presents unexplored, yet valuable opportunities to improve cardiovascular outcomes in this vulnerable patient group.
Keywords: Diabetes; Inflammation; Myocardial infarction; Trained immunity.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: RPC has received payment for consultancy from NovoNordisk, Nodthera, and Velakor.
Figures
References
-
- Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J; Emerging risk factors collaboration . Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841. - PMC - PubMed
-
- Bauters C, Lemesle G, de Groote P, Lamblin N. A systematic review and meta-regression of temporal trends in the excess mortality associated with diabetes mellitus after myocardial infarction. Int J Cardiol 2016;217:109–121. - PubMed
-
- Simek S, Motovska Z, Hlinomaz O, Kala P, Hromadka M, Knot J, Varvarovsky I, Dusek J, Rokyta R, Tousek F, Svoboda M, Vodzinska A, Mrozek J, Jarkovsky J; On behalf of the prague-study group . The effect of diabetes on prognosis following myocardial infarction treated with primary angioplasty and potent antiplatelet therapy. J Clin Med 2020;9:2555. - PMC - PubMed
-
- Ritsinger V, Nyström T, Saleh N, Lagerqvist B, Norhammar A. Heart failure is a common complication after acute myocardial infarction in patients with diabetes: a nationwide study in the SWEDEHEART registry. Eur J Prev Cardiol 2020;27:1890–1901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

