Alzheimer's disease linked Aβ42 exerts product feedback inhibition on γ-secretase impairing downstream cell signaling
- PMID: 39027984
- PMCID: PMC11259434
- DOI: 10.7554/eLife.90690
Alzheimer's disease linked Aβ42 exerts product feedback inhibition on γ-secretase impairing downstream cell signaling
Abstract
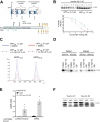
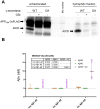
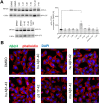
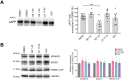
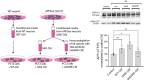
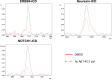
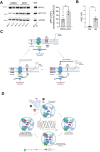
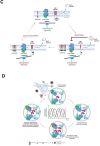
Amyloid β (Aβ) peptides accumulating in the brain are proposed to trigger Alzheimer's disease (AD). However, molecular cascades underlying their toxicity are poorly defined. Here, we explored a novel hypothesis for Aβ42 toxicity that arises from its proven affinity for γ-secretases. We hypothesized that the reported increases in Aβ42, particularly in the endolysosomal compartment, promote the establishment of a product feedback inhibitory mechanism on γ-secretases, and thereby impair downstream signaling events. We conducted kinetic analyses of γ-secretase activity in cell-free systems in the presence of Aβ, as well as cell-based and ex vivo assays in neuronal cell lines, neurons, and brain synaptosomes to assess the impact of Aβ on γ-secretases. We show that human Aβ42 peptides, but neither murine Aβ42 nor human Aβ17-42 (p3), inhibit γ-secretases and trigger accumulation of unprocessed substrates in neurons, including C-terminal fragments (CTFs) of APP, p75, and pan-cadherin. Moreover, Aβ42 treatment dysregulated cellular homeostasis, as shown by the induction of p75-dependent neuronal death in two distinct cellular systems. Our findings raise the possibility that pathological elevations in Aβ42 contribute to cellular toxicity via the γ-secretase inhibition, and provide a novel conceptual framework to address Aβ toxicity in the context of γ-secretase-dependent homeostatic signaling.
Keywords: Alzheimer's disease; amyloid beta; amyloid toxicity; biochemistry; chemical biology; gamma-secretase; gamma-secretase inhibition; human; mouse; neuroscience; presenilin; rat.
© 2023, Zoltowska et al.
Conflict of interest statement
KZ, UD, SL, TE, MM, MH, MF, BÖ, DG, DK, AB, CH, MV, OB, LC No competing interests declared, WM consulted for Samumed and AC immune. He served on advisory boards for the Bluefield Project to Cure Frontotemporal Dementia, the Blythedale-Burke Pediatric Neuroscience Research Collaboration, The Key, the National Down Syndrome Society, the American Neurological Association, the Sanford Health Lorraine Cross Award Committee, the NIH COBRE program at the University of Nebraska, the Dementia Aware Committee and the Dementia Committee for the State of California Health Services, and the San Diego Alzheimer's Project. He was a member of a Pfizer Data Safety Monitoring Board. WM is a co-inventor on UCSD Patents for Gamma-secretase Modulators. WM received grant or contract funding from the NIH, Ono Pharma Foundation, Cure Alzheimer Fund, DH Chen Foundation, AC Immune, Larry L Hillblom Foundation, Alzheimer Association, Annovis-Bio and BioSplice and the Michael J Fox Foundation. He received a travel reimbursement from AC Immune. Annovis Bio provided a gift to the WM lab and a test compound
Figures






Update of
-
Alzheimer's disease linked Aβ42 exerts product feedback inhibition on γ-secretase impairing downstream cell signaling.bioRxiv [Preprint]. 2024 Apr 23:2023.08.02.551596. doi: 10.1101/2023.08.02.551596. bioRxiv. 2024. Update in: Elife. 2024 Jul 19;12:RP90690. doi: 10.7554/eLife.90690. PMID: 37577527 Free PMC article. Updated. Preprint.
References
-
- Acx H, Serneels L, Radaelli E, Muyldermans S, Vincke C, Pepermans E, Müller U, Chávez-Gutiérrez L, De Strooper B. Inactivation of γ-secretases leads to accumulation of substrates and non-Alzheimer neurodegeneration. EMBO Molecular Medicine. 2017;9:1088–1099. doi: 10.15252/emmm.201707561. - DOI - PMC - PubMed
-
- Bahr BA, Hoffman KB, Yang AJ, Hess US, Glabe CG, Lynch G. Amyloid beta protein is internalized selectively by hippocampal field CA1 and causes neurons to accumulate amyloidogenic carboxyterminal fragments of the amyloid precursor protein. The Journal of Comparative Neurology. 1998;397:139–147. - PubMed
-
- Bi HR, Zhou CH, Zhang YZ, Cai XD, Ji MH, Yang JJ, Chen GQ, Hu YM. Neuron-specific deletion of presenilin enhancer2 causes progressive astrogliosis and age-related neurodegeneration in the cortex independent of the Notch signaling. CNS Neuroscience & Therapeutics. 2021;27:174–185. doi: 10.1111/cns.13454. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

