Effect of Intramyocardial Administration of Baculovirus Encoding the Transcription Factor Tbx20 in Sheep With Experimental Acute Myocardial Infarction
- PMID: 39028008
- PMCID: PMC11964036
- DOI: 10.1161/JAHA.123.031515
Effect of Intramyocardial Administration of Baculovirus Encoding the Transcription Factor Tbx20 in Sheep With Experimental Acute Myocardial Infarction
Abstract
Background: Gene therapy has been proposed as a strategy to induce cardiac regeneration following acute myocardial infarction (AMI). Given that Tbx20, a transcription factor of the T-box subfamily, stimulates cell proliferation and angiogenesis, we designed a baculovirus overexpressing Tbx20 (Bv-Tbx20) and evaluated its effects in cultured cardiomyocytes and in an ovine model of AMI.
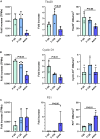
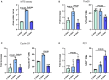
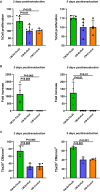
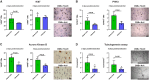
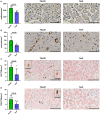
Methods and results: Cell proliferation and angiogenesis were measured in cardiomyocytes transduced with Bv-Tbx20 or Bv-Null (control). Subsequently, in sheep with AMI, Bv-Tbx20 or Bv-Null was injected in the infarct border. Cardiomyocyte cell cycle activity, angioarteriogenesis, left ventricular function, and infarct size were assessed. Cardiomyocytes transduced with BvTbx20 increased cell proliferation, cell cycle regulatory and angiogenic gene expression, and tubulogenesis. At 7 days posttreatment, sheep treated with Bv-Tbx20 showed increased Tbx20, promitotic and angiogenic gene expression, decreased levels of P21, increased Ki67- (17.09±5.73 versus 7.77±7.24 cardiomyocytes/mm2, P<0.05) and PHH3 (phospho-histone H3)-labeled cardiomyocytes (10.10±3.51 versus 5.23±2.87 cardiomyocytes/mm2, P<0.05), and increased capillary (2302.68±353.58 versus 1694.52±211.36 capillaries/mm2, P<0.001) and arteriolar (146.95±53.14 versus 84.06±16.84 arterioles/mm2, P<0.05) densities. At 30 days, Bv-Tbx20 decreased infarct size (9.89±1.92% versus 12.62±1.33%, P<0.05) and slightly improved left ventricular function. Baculoviral gene transfer-mediated Tbx20 overexpression exerted angiogenic and cardiomyogenic effects in vitro.
Conclusions: In sheep with AMI, Bv-Tbx20 induced angioarteriogenesis, cardiomyocyte cell cycle activity, infarct size limitation, and a slight recovery of left ventricular function, suggesting that Bv-Tbx20 gene therapy may contribute to cardiac regeneration following AMI.
Keywords: Tbx20; acute myocardial infarction; baculovirus; gene therapy; sheep.
Figures

References
-
- Stennard FA, Costa MW, Elliott DA, Rankin S, Haast SJ, Lai D, McDonald LP, Niederreither K, Dolle P, Bruneau BG, et al. Cardiac T‐box factor Tbx20 directly interacts with Nkx2‐5, GATA4, and GATA5 in regulation of gene expression in the developing heart. Dev Biol. 2004;262:206–224. doi: 10.1016/S0012-1606(03)00385-3 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

