Modulation of the high concentration viscosity of IgG1 antibodies using clinically validated Fc mutations
- PMID: 39028186
- PMCID: PMC11262234
- DOI: 10.1080/19420862.2024.2379560
Modulation of the high concentration viscosity of IgG1 antibodies using clinically validated Fc mutations
Abstract
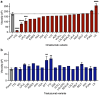
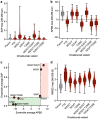
The self-association of therapeutic antibodies can result in elevated viscosity and create problems in manufacturing and formulation, as well as limit delivery by subcutaneous injection. The high concentration viscosity of some antibodies has been reduced by variable domain mutations or by the addition of formulation excipients. In contrast, the impact of Fc mutations on antibody viscosity has been minimally explored. Here, we studied the effect of a panel of common and clinically validated Fc mutations on the viscosity of two closely related humanized IgG1, κ antibodies, omalizumab (anti-IgE) and trastuzumab (anti-HER2). Data presented here suggest that both Fab-Fab and Fab-Fc interactions contribute to the high viscosity of omalizumab, in a four-contact model of self-association. Most strikingly, the high viscosity of omalizumab (176 cP) was reduced 10.7- and 2.2-fold by Fc modifications for half-life extension (M252Y:S254T:T256E) and aglycosylation (N297G), respectively. Related single mutations (S254T and T256E) each reduced the viscosity of omalizumab by ~6-fold. An alternative half-life extension Fc mutant (M428L:N434S) had the opposite effect in increasing the viscosity of omalizumab by 1.5-fold. The low viscosity of trastuzumab (8.6 cP) was unchanged or increased by 2-fold by the different Fc variants. Molecular dynamics simulations provided mechanistic insight into the impact of Fc mutations in modulating electrostatic and hydrophobic surface properties as well as conformational stability of the Fc. This study demonstrates that high viscosity of some IgG1 antibodies can be mitigated by Fc mutations, and thereby offers an additional tool to help design future antibody therapeutics potentially suitable for subcutaneous delivery.
Keywords: Intermolecular interactions; intramolecular interactions; molecular dynamics; rheology; self-association; subcutaneous delivery; viscosity.
Conflict of interest statement
All authors are current employees of Genentech, Inc., which develops and commercializes therapeutics including antibodies.
Figures






References
-
- The Antibody Society. Therapeutic monoclonal antibodies approved or in regulatory review . accessed 2024 May 3]. www.antibodysociety.org/antibody-therapeutics-product-data.
-
- Ghosh I, Gutka H, Krause ME, Clemens R, Kashi RS.. A systematic review of commercial high concentration antibody drug products approved in the US: formulation composition, dosage form design and primary packaging considerations. MAbs. 2023;15(1):2205540. doi:10.1080/19420862.2023.2205540. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
