A Randomised Controlled Trial of SFX-01 After Subarachnoid Haemorrhage - The SAS Study
- PMID: 39028412
- PMCID: PMC12202693
- DOI: 10.1007/s12975-024-01278-1
A Randomised Controlled Trial of SFX-01 After Subarachnoid Haemorrhage - The SAS Study
Abstract
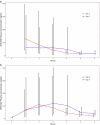
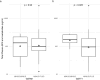
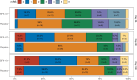
SFX-01 is a novel drug for clinical delivery of sulforaphane (SFN). SFN is a potent nuclear factor erythroid 2-related factor 2 activator that reduces inflammation and oxidation, improving outcomes after subarachnoid haemorrhage (SAH) in animal models. This was a multi-centre, double-blind, placebo-controlled, parallel-group randomised clinical trial to evaluate the safety, pharmacokinetics and efficacy of 28 days of SFX-01 300 mg BD in patients aged 18-80 with spontaneous SAH and high blood load on CT. Primary outcomes were (1) safety, (2) plasma and CSF SFN and metabolite levels and (3) vasospasm on transcranial doppler ultrasound. Secondary outcomes included CSF haptoglobin and malondialdehyde and clinical outcome on the modified Rankin Scale (mRS) and SAH outcome tool (SAHOT). A total of 105 patients were randomised (54 SFX-01, 51 placebo). There were no differences in adverse events other than nausea (9 SFX-01 (16.7%), 1 placebo (2.0%)). SFN, SFN-glutathione and SFN-N-acetyl-cysteine AUClast were 16.2, 277 and 415 h × ng/ml. Plasma SFN was higher in GSTT1 null individuals (t = 2.40, p = 0.023). CSF levels were low with many samples below the lower limit of quantification and predicted by the CSF/serum albumin ratio (R2 = 0.182, p = 0.039). There was no difference in CSF haptoglobin (1.981 95%CI 0.992-3.786, p = 0.052) or malondialdehyde (1.12 95%CI 0.7477-1.687, p = 0.572) or middle cerebral artery flow velocity (1.04 95%CI 0.903-1.211, p = 0.545) or functional outcome (mRS 1.647 95%CI 0.721-3.821, p = 0.237, SAHOT 1.082 95%CI 0.464-2.525, p = 0.855). SFX-01 is safe and effective for the delivery of SFN in acutely unwell patients. SFN penetrated CSF less than expected and did not reduce large vessel vasospasm or improve outcome. Trial registration: NCT02614742 clinicaltrials.gov.
Keywords: Haptoglobin; Nrf2; Pharmacokinetics; Randomised clinical trial; Subarachnoid haemorrhage; Sulforaphane.
© 2024. Crown.
Conflict of interest statement
Declarations. Ethical Approval: The trial was approved by the National Research Ethics Service (Southern Central Hampshire A) and Medicinal Health Care Authority (MHRA) and was conducted in accordance with the Declaration of Helsinki and met the international criteria for Good Clinical Practice. Informed consents were obtained from patients or legal representatives. The trial was registered on clinicaltrials.gov (NCT02614742) and the Consolidated Standards of Reporting Trials (CONSORT) was followed. Conflict of Interest: Diederik Bulters and Ian Galea have received support for their research from BPL and CSL Behring. David Howatt and Stephen Franklin were employees of Evgen Pharma. Patrick Garland is employed by Kedrion.
Figures



References
-
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–22. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
