Genetic testing in early-onset atrial fibrillation
- PMID: 39028637
- PMCID: PMC11379493
- DOI: 10.1093/eurheartj/ehae298
Genetic testing in early-onset atrial fibrillation
Abstract
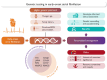
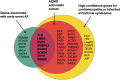
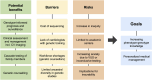
Atrial fibrillation (AF) is a globally prevalent cardiac arrhythmia with significant genetic underpinnings, as highlighted by recent large-scale genetic studies. A prominent clinical and genetic overlap exists between AF, heritable ventricular cardiomyopathies, and arrhythmia syndromes, underlining the potential of AF as an early indicator of severe ventricular disease in younger individuals. Indeed, several recent studies have demonstrated meaningful yields of rare pathogenic variants among early-onset AF patients (∼4%-11%), most notably for cardiomyopathy genes in which rare variants are considered clinically actionable. Genetic testing thus presents a promising opportunity to identify monogenetic defects linked to AF and inherited cardiac conditions, such as cardiomyopathy, and may contribute to prognosis and management in early-onset AF patients. A first step towards recognizing this monogenic contribution was taken with the Class IIb recommendation for genetic testing in AF patients aged 45 years or younger by the 2023 American College of Cardiology/American Heart Association guidelines for AF. By identifying pathogenic genetic variants known to underlie inherited cardiomyopathies and arrhythmia syndromes, a personalized care pathway can be developed, encompassing more tailored screening, cascade testing, and potentially genotype-informed prognosis and preventive measures. However, this can only be ensured by frameworks that are developed and supported by all stakeholders. Ambiguity in test results such as variants of uncertain significance remain a major challenge and as many as ∼60% of people with early-onset AF might carry such variants. Patient education (including pretest counselling), training of genetic teams, selection of high-confidence genes, and careful reporting are strategies to mitigate this. Further challenges to implementation include financial barriers, insurability issues, workforce limitations, and the need for standardized definitions in a fast-moving field. Moreover, the prevailing genetic evidence largely rests on European descent populations, underscoring the need for diverse research cohorts and international collaboration. Embracing these challenges and the potential of genetic testing may improve AF care. However, further research-mechanistic, translational, and clinical-is urgently needed.
Keywords: Atrial fibrillation; Cardiomyopathy; Cascade testing; Genetic testing; Rare variants.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Figures

References
Publication types
MeSH terms
Grants and funding
- NNF17OC0031204/Hallas-Møller Emerging Investigator Novo Nordisk Fonden
- New South Wales Health Cardiovascular Disease Clinician Scientist
- EU IMI 116074/BigData@Heart
- 81Z1710103/German Center for Cardiovascular Research
- ZONMW_/ZonMw/Netherlands
- Ministry of Health
- #2016822/National Health and Medical Research Council
- BMBF 01ZX1408A/German Ministry of Research and Education
- R01 HL139731/HL/NHLBI NIH HHS/United States
- 965286/Leducq Foundation
- U24 HG006834/HG/NHGRI NIH HHS/United States
- R01HL092577/NH/NIH HHS/United States
- R01 HL155197/HL/NHLBI NIH HHS/United States
- R01 HL092577/HL/NHLBI NIH HHS/United States
- 031L0239/ERACoSysMed3
- 03-007-2022-0035/Dutch Heart Foundation
- AHA_18SFRN34110082/AHA/American Heart Association-American Stroke Association/United States
- FS/13/43/30324/BHF_/British Heart Foundation/United Kingdom
- U24HG006834/HG/NHGRI NIH HHS/United States
- ERC_/European Research Council/International
- Walter Benjamin Fellowship
- 633196/European Union
- German Heart Foundation
- DZHK FKZ 81X2800182/German Ministry of Education and Research
- R01 HL157635/HL/NHLBI NIH HHS/United States
- 648131/European Union's Horizon 2020
- 521832260/Deutsche Forschungsgemeinschaft
LinkOut - more resources
Full Text Sources
Medical

