HIV-1 transmitted drug resistance in newly diagnosed individuals in Italy over the period 2015-21
- PMID: 39028674
- PMCID: PMC11368429
- DOI: 10.1093/jac/dkae189
HIV-1 transmitted drug resistance in newly diagnosed individuals in Italy over the period 2015-21
Abstract
Background: Transmitted drug resistance (TDR) is still a critical aspect for the management of individuals living with HIV-1. Thus, its evaluation is crucial to optimize HIV care.
Methods: Overall, 2386 HIV-1 protease/reverse transcriptase and 1831 integrase sequences from drug-naïve individuals diagnosed in north and central Italy between 2015 and 2021 were analysed. TDR was evaluated over time. Phylogeny was generated by maximum likelihood. Factors associated with TDR were evaluated by logistic regression.
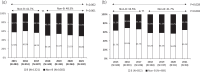
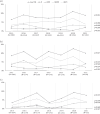
Results: Individuals were mainly male (79.1%) and Italian (56.2%), with a median (IQR) age of 38 (30-48). Non-B infected individuals accounted for 44.6% (N = 1065) of the overall population and increased over time (2015-2021, from 42.1% to 51.0%, P = 0.002). TDR prevalence to any class was 8.0% (B subtype 9.5% versus non-B subtypes 6.1%, P = 0.002) and remained almost constant over time. Overall, 300 transmission clusters (TCs) involving 1155 (48.4%) individuals were identified, with a similar proportion in B and non-infected individuals (49.7% versus 46.8%, P = 0.148). A similar prevalence of TDR among individuals in TCs and those out of TCs was found (8.2% versus 7.8%, P = 0.707).By multivariable analysis, subtypes A, F, and CFR02_AG were negatively associated with TDR. No other factors, including being part of TCs, were significantly associated with TDR.
Conclusions: Between 2015 and 2021, TDR prevalence in Italy was 8% and remained almost stable over time. Resistant strains were found circulating regardless of being in TCs, but less likely in non-B subtypes. These results highlight the importance of a continuous surveillance of newly diagnosed individuals for evidence of TDR to inform clinical practice.
© The Author(s) 2024. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures


References
-
- Wittkop L, Günthard HF, de Wolf Fet al. . Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN Joint Project): a European Multicohort Study. Lancet Infect Dis 2011; 11: 363–71. 10.1016/S1473-3099(11)70032-9 - DOI - PubMed
-
- European AIDS Clinical Society (EACS) . EACS Guidelines, Version 12.0, 2023. https://www.eacsociety.org/guidelines/eacs-guidelines/
-
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV Developed by the DHHS Panel on Antiretroviral Guidelines for Adults, 2024. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/ad...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

