Cumulative donor-specific antibody threshold predicts platelet transfusion response in HLA-alloimmunized patients
- PMID: 39028936
- PMCID: PMC11402140
- DOI: 10.1182/bloodadvances.2024014143
Cumulative donor-specific antibody threshold predicts platelet transfusion response in HLA-alloimmunized patients
Abstract
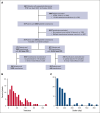
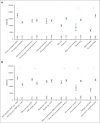
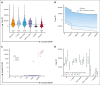
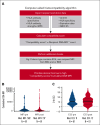
Up to a third of patients with hemato-oncologic conditions who have received multiply transfusions develop immune-mediated platelet transfusion refractoriness. Yet factors that influence posttransfusion platelet corrected count increments (CCI) in patients with HLA-alloimmune platelet transfusion refractoriness remain less well elucidated. Recent advances in HLA antibody characterization using fluorescent bead-based platforms enable the study of donor-specific antibody (DSA) avidity (as measured by mean fluorescence intensity [MFI]) and its impact on HLA-alloimmune platelet transfusion refractoriness. In this large retrospective study of 2012 platelet transfusions among 73 HLA-alloimmunized patients, we evaluated the impact of cumulative HLA DSA-MFI alongside other donor, platelet component, and patient characteristics on CCI at 2 and 24 hours after transfusion. As part of a quality improvement initiative, we also developed and tested a computerized algorithm to optimize donor-recipient histocompatibility based on cumulative DSA-MFI and sought other actionable predictors of CCI. In multivariate analyses, cumulative HLA DSA-MFI of ≥10 000, major/bidirectional ABO-mismatch, splenomegaly, transfusion reactions, and platelet storage in additive solution negatively affected 2-hour but not 24-hour posttransfusion CCI. The DSA-MFI threshold of 10 000 was corroborated by greater antibody-mediated complement activation and significantly more CCI failures above this threshold, suggesting the usefulness of this value to inform "permissive platelet mismatching" and to optimize CCI. Furthermore, DSA-MFI decreases were deemed feasible by the computer-based algorithm for HLA-platelet selection in a pilot cohort of 8 patients (122 transfusions) evaluated before and after algorithm implementation. When HLA-selected platelets are unavailable, ABO-identical/minor-mismatched platelet concentrates may enhance 2-hour CCI in heavily HLA-alloimmunized patients with platelet transfusion refractoriness.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: S.R.P. reports consultancy fees from Sanofi Inc and Sobi Inc. L.S.-H. reports personal fees and nonfinancial support from the Institute for Healthcare Improvement, outside the submitted work. The remaining authors declare no competing financial interests.
Figures

References
-
- Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol. 2008;142(3):348–360. - PubMed
-
- Yankee RA, Grumet FC, Rogentine GN. Platelet transfusion therapy; the selection of compatible platelet donors for refractory patients by lymphocyte HL-A typing. N Engl J Med. 1969;281(22):1208–1212. - PubMed
-
- Petz LD, Garratty G, Calhoun L, et al. Selecting donors of platelets for refractory patients on the basis of HLA antibody specificity. Transfusion. 2000;40(12):1446–1456. - PubMed
-
- Vassallo RR, Fung M, Rebulla P, et al. Utility of cross-matched platelet transfusions in patients with hypoproliferative thrombocytopenia: a systematic review. Transfusion. 2014;54(4):1180–1191. - PubMed
-
- Duquesnoy RJ, Filip DJ, Rodey GE, Rimm AA, Aster RH. Successful transfusion of platelets "mismatched" for HLA antigens to alloimmunized thrombocytopenic patients. Am J Hematol. 1977;2(3):219–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

