Inhibition of ferroptosis rescues M2 macrophages and alleviates arthritis by suppressing the HMGB1/TLR4/STAT3 axis in M1 macrophages
- PMID: 39029270
- PMCID: PMC11304870
- DOI: 10.1016/j.redox.2024.103255
Inhibition of ferroptosis rescues M2 macrophages and alleviates arthritis by suppressing the HMGB1/TLR4/STAT3 axis in M1 macrophages
Abstract
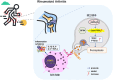
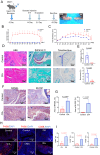
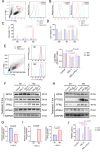
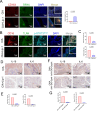
Ferroptosis is a type of programmed cell death driven by iron-dependent lipid peroxidation. The TNF-mediated biosynthesis of glutathione has been shown to protect synovial fibroblasts from ferroptosis in the hyperplastic synovium. Ferroptosis induction provides a novel therapeutic approach for rheumatoid arthritis (RA) by reducing the population of synovial fibroblasts. The beginning and maintenance of synovitis in RA are significantly influenced by macrophages, as they generate cytokines that promote inflammation and contribute to the destruction of cartilage and bone. However, the vulnerability of macrophages to ferroptosis in RA remains unclear. In this study, we found that M2 macrophages are more vulnerable to ferroptosis than M1 macrophages in the environment of the arthritis synovium with a high level of iron, leading to an imbalance in the M1/M2 ratio. During ferroptosis, HMGB1 released by M2 macrophages interacts with TLR4 on M1 macrophages, which in turn triggers the activation of STAT3 signaling in M1 macrophages and contributes to the inflammatory response. Knockdown of TLR4 decreased the level of cytokines induced by HMGB1 in M1 macrophages. The ferroptosis inhibitor liproxstatin-1 (Lip-1) started at the presymptomatic stage in collagen-induced arthritis (CIA) model mice, and GPX4 overexpression in M2 macrophages at the onset of collagen antibody-induced arthritis (CAIA) protected M2 macrophages from ferroptotic cell death and significantly prevented the development of joint inflammation and destruction. Thus, our study demonstrated that M2 macrophages are vulnerable to ferroptosis in the microenvironment of the hyperplastic synovium and revealed that the HMGB1/TLR4/STAT3 axis is critical for the ability of ferroptotic M2 macrophages to contribute to the exacerbation of synovial inflammation in RA. Our findings provide novel insight into the progression and treatment of RA.
Keywords: Ferroptosis; Macrophage; Rheumatoid arthritis.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no conflict of interest relevant to this study to declare.
Figures



References
-
- Chauhan K., Jandu J.S., Brent L.H., Al-Dhahir M.A. StatPearls. 2024. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

