Human translesion DNA polymerases ι and κ mediate tolerance to temozolomide in MGMT-deficient glioblastoma cells
- PMID: 39029375
- PMCID: PMC11330349
- DOI: 10.1016/j.dnarep.2024.103715
Human translesion DNA polymerases ι and κ mediate tolerance to temozolomide in MGMT-deficient glioblastoma cells
Abstract
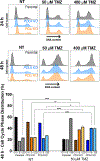
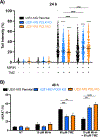
Glioblastoma (GBM) is a highly aggressive brain tumor associated with poor patient survival. The current standard treatment involves invasive surgery, radiotherapy, and chemotherapy employing temozolomide (TMZ). Resistance to TMZ is, however, a major challenge. Previous work from our group has identified candidate genes linked to TMZ resistance, including genes encoding translesion synthesis (TLS) DNA polymerases iota (Polɩ) and kappa (Polκ). These specialized enzymes are known for bypassing lesions and tolerating DNA damage. Here, we investigated the roles of Polɩ and Polκ in TMZ resistance, employing MGMT-deficient U251-MG glioblastoma cells, with knockout of either POLI or POLK genes encoding Polɩ and Polκ, respectively, and assess their viability and genotoxic stress responses upon subsequent TMZ treatment. Cells lacking either of these polymerases exhibited a significant decrease in viability following TMZ treatment compared to parental counterparts. The restoration of the missing polymerase led to a recovery of cell viability. Furthermore, knockout cells displayed increased cell cycle arrest, mainly in late S-phase, and lower levels of genotoxic stress after TMZ treatment, as assessed by a reduction of γH2AX foci and flow cytometry data. This implies that TMZ treatment does not trigger a significant H2AX phosphorylation response in the absence of these proteins. Interestingly, combining TMZ with Mirin (double-strand break repair pathway inhibitor) further reduced the cell viability and increased DNA damage and γH2AX positive cells in TLS KO cells, but not in parental cells. These findings underscore the crucial roles of Polɩ and Polκ in conferring TMZ resistance and the potential backup role of homologous recombination in the absence of these TLS polymerases. Targeting these TLS enzymes, along with double-strand break DNA repair inhibition, could, therefore, provide a promising strategy to enhance TMZ's effectiveness in treating GBM.
Keywords: Cancer; Chemotherapy resistance; DNA polymerase; Glioma; Temozolomide; Translesion synthesis.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest CFMM is an Editorial Board Member of DNA Repair. RW is an Associate Editor of DNA Repair. Neither were involved in the editorial review or the decision to publish this article.
Figures







References
-
- Kannouche PL, Lehmann AR, Ubiquitination of PCNA and the polymerase switch in human cells, Cell Cycle, 3 (2004) 1011–1013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

