Intestinal microbiota composition is predictive of radiotherapy-induced acute gastrointestinal toxicity in prostate cancer patients
- PMID: 39029427
- PMCID: PMC11314862
- DOI: 10.1016/j.ebiom.2024.105246
Intestinal microbiota composition is predictive of radiotherapy-induced acute gastrointestinal toxicity in prostate cancer patients
Abstract
Background: The search for factors beyond the radiotherapy dose that could identify patients more at risk of developing radio-induced toxicity is essential to establish personalised treatment protocols for improving the quality-of-life of survivors. To investigate the role of the intestinal microbiota in the development of radiotherapy-induced gastrointestinal toxicity, the MicroLearner observational cohort study characterised the intestinal microbiota of 136 (discovery) and 79 (validation) consecutive prostate cancer patients at baseline radiotherapy.
Methods: Gastrointestinal toxicity was assessed weekly during RT using CTCAE. An average grade >1.3 over time points was used to identify patients suffering from persistent acute toxicity (endpoint). The microbiota of patients was quantified from the baseline faecal samples using 16S rRNA gene sequencing technology and the Ion Reporter metagenomic pipeline. Statistical techniques and computational and machine learning tools were used to extract, functionally characterise, and predict core features of the bacterial communities of patients who developed acute gastrointestinal toxicity.
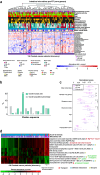
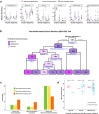
Findings: Analysis of the core bacterial composition in the discovery cohort revealed a cluster of patients significantly enriched for toxicity, displaying a toxicity rate of 60%. Based on selected high-risk microbiota compositional features, we developed a clinical decision tree that could effectively predict the risk of toxicity based on the relative abundance of genera Faecalibacterium, Bacteroides, Parabacteroides, Alistipes, Prevotella and Phascolarctobacterium both in internal and external validation cohorts.
Interpretation: We provide evidence showing that intestinal bacteria profiling from baseline faecal samples can be effectively used in the clinic to improve the pre-radiotherapy assessment of gastrointestinal toxicity risk in prostate cancer patients.
Funding: Italian Ministry of Health (Promotion of Institutional Research INT-year 2016, 5 × 1000, Ricerca Corrente funds). Fondazione Regionale per la Ricerca Biomedica (ID 2721017). AIRC (IG 21479).
Keywords: Intestinal microbiota; Machine learning; Prostate cancer; Radiation toxicity.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no conflict of interest.
Figures

References
-
- Budäus L., Bolla M., Bossi A., et al. Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of the literature. Eur Urol. 2012;61(1):112–127. - PubMed
-
- Heemsbergen W.D., Peeters S.T.H., Koper P.C.M., Hoogeman M.S., Lebesque J.V. Acute and late gastrointestinal toxicity after radiotherapy in prostate cancer patients: consequential late damage. Int J Radiat Oncol Biol Phys. 2006;66(1):3–10. - PubMed
-
- Ferreira M.R., Muls A., Dearnaley D.P., Andreyev H.J.N. Microbiota and radiation-induced bowel toxicity: lessons from inflammatory bowel disease for the radiation oncologist. Lancet Oncol. 2014;15(3):e139–e147. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

