Pretreatment CT-based machine learning radiomics model predicts response in unresectable hepatocellular carcinoma treated with lenvatinib plus PD-1 inhibitors and interventional therapy
- PMID: 39029924
- PMCID: PMC11261678
- DOI: 10.1136/jitc-2024-008953
Pretreatment CT-based machine learning radiomics model predicts response in unresectable hepatocellular carcinoma treated with lenvatinib plus PD-1 inhibitors and interventional therapy
Abstract
Background: Lenvatinib plus PD-1 inhibitors and interventional (LPI) therapy have demonstrated promising treatment effects in unresectable hepatocellular carcinoma (HCC). However, biomarkers for predicting the response to LPI therapy remain to be further explored. We aimed to develop a radiomics model to noninvasively predict the efficacy of LPI therapy.
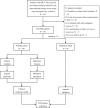
Methods: Clinical data of patients with HCC receiving LPI therapy were collected in our institution. The clinical model was built with clinical information. Nine machine learning classifiers were tested and the multilayer perceptron classifier with optimal performance was used as the radiomics model. The clinical-radiomics model was constructed by integrating clinical and radiomics scores through logistic regression analysis.
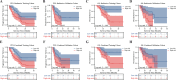
Results: 151 patients were enrolled in this study (2:1 randomization, 101 and 50 in the training and validation cohorts), of which three achieved complete response, 69 showed partial response, 46 showed stable disease, and 33 showed progressive disease. The objective response rate, disease control rate, and conversion resection rates were 47.7, 78.1 and 23.2%. 14 features were selected from the initially extracted 1223 for radiomics model construction. The area under the curves of the radiomics model (0.900 for training and 0.893 for validation) were comparable to that of the clinical-radiomics model (0.912 for training and 0.892 for validation), and both were superior to the clinical model (0.669 for training and 0.585 for validation). Meanwhile, the radiomics model can categorize participants into high-risk and low-risk groups for progression-free survival (PFS) and overall survival (OS) in the training (HR 1.913, 95% CI 1.121 to 3.265, p=0.016 for PFS; HR 4.252, 95% CI 2.051 to 8.816, p=0.001 for OS) and validation sets (HR 2.347, 95% CI 1.095 to 5.031, p=0.012 for PFS; HR 2.592, 95% CI 1.050 to 6.394, p=0.019 for OS).
Conclusion: The promising machine learning radiomics model was developed and validated to predict the efficacy of LPI therapy for patients with HCC and perform risk stratification, with comparable performance to clinical-radiomics model.
Keywords: Biomarker; Combination therapy; Hepatocellular Carcinoma; Immune Checkpoint Inhibitor.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
