Generation of non-genetically modified, CAR-like, NK cells
- PMID: 39029925
- PMCID: PMC11261687
- DOI: 10.1136/jitc-2024-009070
Generation of non-genetically modified, CAR-like, NK cells
Abstract
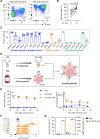
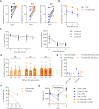
Background: Natural killer (NK) cell therapy is considered an attractive and safe strategy for anticancer therapy. Nevertheless, when autologous or allogenic NK cells are used alone, the clinical benefit has been disappointing. This is partially due to the lack of target specificity. Recently, CD19-specific chimeric antigen receptor (CAR)-NK cells have proven to be safe and potent in patients with B-cell tumors. However, the generation of CAR-NK cells is a complicated manufacturing process. We aim at developing a targeted NK cell therapy without the need for cellular genetic modifications. We took advantage of the natural expression of the IgG Fc receptor CD16a (FcγRIIIa) to induce strong antigen-specific effector functions through antibody-dependent cell-mediated cytotoxicity (ADCC). We have generated the new technology "Pin", which enables the arming of modified monoclonal antibodies (mAbs) onto the CD16a of ex vivo expanded NK (eNK) cells. Methods Ex vivo eNK were prepared from umbilical cord blood cells and expanded using interleukin (IL)-2/IL-15 and Epstein-Barr virus (EBV)-transformed B-lymphoblastoid feeder cells. mAbs were engineered with four substitutions called Pin mutations to increase their affinity to CD16a. eNK were incubated with anti-CD20 or anti-CD19 Pin-mAbs to generate "armed" eNK and were used to assess effector functions in vitro on cancer cell lines, lymphoma patient cells and in vivo.
Results: CD16a/Pin-mAb interaction is stable for several days and Pin-mAb eNK inherit the mAb specificity and exclusively induce ADCC against targets expressing the cognate antigen. Hence, Pin-mAbs confer long-term selectivity to eNK, which allows specific elimination of the target cells in several in vivo mouse models. Finally, we showed that it is possible to arm eNK with at least two Pin-mAbs simultaneously, to increase efficacy against heterogenous cancer cell populations.
Conclusions: The Pin technology provides an off-the-shelf NK cell therapy platform to generate CAR-like NK cells, without genetic modifications, that easily target multiple tumor antigens.
Keywords: Adoptive cell therapy - ACT; Immunotherapy; Monoclonal antibody; Natural killer - NK.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The patent of PINTM technology has been licensed to CYTEA BIO (WO2022023581A1 “Armed NK cells for universal cell therapy”). ER, HC, BF and JP are currently employees of CYTEA BIO. BR, PM and MV were initial creators of CYTEA BIO.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
