Whole cervix imaging of collagen, muscle, and cellularity in term and preterm pregnancy
- PMID: 39030173
- PMCID: PMC11271604
- DOI: 10.1038/s41467-024-48680-9
Whole cervix imaging of collagen, muscle, and cellularity in term and preterm pregnancy
Abstract
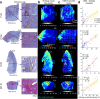
Cervical softening and dilation are critical for the successful term delivery of a fetus, with premature changes associated with preterm birth. Traditional clinical measures like transvaginal ultrasound and Bishop scores fall short in predicting preterm births and elucidating the cervix's complex microstructural changes. Here, we introduce a magnetic resonance diffusion basis spectrum imaging (DBSI) technique for non-invasive, comprehensive imaging of cervical cellularity, collagen, and muscle fibers. This method is validated through ex vivo DBSI and histological analyses of specimens from total hysterectomies. Subsequently, retrospective in vivo DBSI analysis at 32 weeks of gestation in ten term deliveries and seven preterm deliveries with inflammation-related conditions shows distinct microstructural differences between the groups, alongside significant correlations with delivery timing. These results highlight DBSI's potential to improve understanding of premature cervical remodeling and aid in the evaluation of therapeutic interventions for at-risk pregnancies. Future studies will further assess DBSI's clinical applicability.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
- 22-FY14-486/March of Dimes Foundation (March of Dimes)
- R03 AG072375/AG/NIA NIH HHS/United States
- R01 HD094381/HD/NICHD NIH HHS/United States
- R01HD094381/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- NGP10119/Burroughs Wellcome Fund (BWF)
- INV-005417, INV-035476, INV-037302/Bill and Melinda Gates Foundation (Bill & Melinda Gates Foundation)
- R03AG072375-01, R01AG074909/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 AG074909/AG/NIA NIH HHS/United States
- R01HD104822/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- R01 HD104822/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

