Electron iso-density surfaces provide a thermodynamically consistent representation of atomic and molecular surfaces
- PMID: 39030194
- PMCID: PMC11271626
- DOI: 10.1038/s41467-024-50408-8
Electron iso-density surfaces provide a thermodynamically consistent representation of atomic and molecular surfaces
Abstract
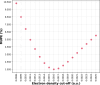
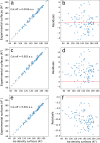
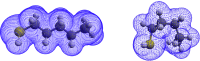
The surface area of atoms and molecules plays a crucial role in shaping many physiochemical properties of materials. Despite its fundamental importance, precisely defining atomic and molecular surfaces has long been a puzzle. Among the available definitions, a straightforward and elegant approach by Bader describes a molecular surface as an iso-density surface beyond which the electron density drops below a certain cut-off. However, so far neither this theory nor a decisive value for the density cut-off have been amenable to experimental verification due to the limitations of conventional experimental methods. In the present study, we employ a state-of-the-art experimental method based on the recently developed concept of thermodynamically effective (TE) surfaces to tackle this longstanding problem. By studying a set of 104 molecules, a close to perfect agreement between quantum chemical evaluations of iso-density surfaces contoured at a cut-off density of 0.0016 a.u. and experimental results obtained via thermodynamic phase change data is demonstrated, with a mean unsigned percentage deviation of 1.6% and a correlation coefficient of 0.995. Accordingly, we suggest the iso-density surface contoured at an electron density value of 0.0016 a.u. as a representation of the surface of atoms and molecules.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Herbert, J. M. Dielectric continuum methods for quantum chemistry. WIREs Comput. Mol. Sci.11, e1519 (2021).10.1002/wcms.1519 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

