Renal and multisystem effectiveness of 3.9 years of migalastat in a global real-world cohort: Results from the followME Fabry Pathfinders registry
- PMID: 39031114
- PMCID: PMC11730455
- DOI: 10.1002/jimd.12771
Renal and multisystem effectiveness of 3.9 years of migalastat in a global real-world cohort: Results from the followME Fabry Pathfinders registry
Abstract
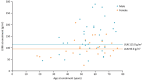
Fabry disease is a progressive, X-linked lysosomal disorder caused by reduced or absent α-galactosidase A activity due to GLA variants. The effects of migalastat were examined in a cohort of 125 Fabry patients with migalastat-amenable GLA variants in the followME Pathfinders registry (EUPAS20599), an ongoing, prospective, patient-focused registry evaluating outcomes for current Fabry disease treatments. We report annualised estimated glomerular filtration rate (eGFR) and Fabry-associated clinical events (FACEs) in a cohort of patients who had received ≥3 years of migalastat treatment in a real-world setting. As of August 2022, 125 patients (60% male) had a mean migalastat exposure of 3.9 years. At enrolment, median age was 58 years (males, 57; females, 60) with a mean eGFR of 83.7 mL/min/1.73 m2 (n = 122; males, 83.7; females, 83.8) and a median left ventricular mass index of 115.1 g/m2 (n = 61; males, 131.2; females, 98.0). Mean (95% confidence interval) eGFR annualised rate of change in the overall cohort (n = 116) was -0.9 (-10.8, 9.9) mL/min/1.73 m2/year with a similar rate of change observed across patients with varying levels of kidney function at enrolment. Despite population age and baseline morbidity, 80% of patients did not experience a FACE during the mean 3.9 years of migalastat exposure. The incidence of renal, cardiac, and cerebrovascular events was 2.0, 83.2, and 4.1 events per 1000 patient-years, respectively. These data support a role of migalastat in preserving renal function and multisystem effectiveness during ≥3 years of migalastat treatment in this real-world Fabry population.
Keywords: Fabry disease; migalastat; real world evidence.
© 2024 The Author(s). Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Derralynn A. Hughes reports grants from Amicus Therapeutics, Inc., consulting fees from Amicus Therapeutics, Inc., Freeline, Idorsia, Protalix/Chiesi, Sanofi, Takeda and Sangamo Therapeutics, Inc., honoraria from Amicus Therapeutics, Inc., Freeline, Idorsia, Protalix/Chiesi, Sanofi and Takeda, and other support from Chiesi, Freeline, Sanofi, Takeda and Amicus Therapeutics, Inc. Gere Sunder‐Plassmann reports advisory board participation for Amicus Therapeutics, Inc., Chiesi and Sanofi, research grants/funding from Amicus Therapeutics, Freeline, Idorsia and Takeda, honoraria from Amicus Therapeutics, Inc., Chiesi, and Sanofi, and other financial support from Amicus Therapeutics, Inc., Chiesi and Sanofi. Ana Jovanovic reports advisory board participation from Amicus Therapeutics, Inc. and Sanofi, research grants and honoraria from Amicus Therapeutics, Inc. and Takeda, and other financial support from Amicus Therapeutics, Inc. Eva Brand reports research grants, consulting fees, advisory board and speaker honoraria from Amicus Therapeutics, Inc., Chiesi, Sanofi Genzyme and Takeda. Michael L. West reports research grants from Takeda, Sanofi, Alexion, Chiesi and AvroBio, consulting fees from Takeda, advisory board participation for Amicus Therapeutics, Inc. and Sanofi, leadership or fiduciary board/committee roles for Sanofi and Takeda, honoraria from Takeda, Sanofi, Sumitomo and Amicus Therapeutics, Inc., payment for expert testimony from Takeda and Amicus Therapeutics, Inc., and other financial support from Amicus Therapeutics, Inc. Daniel G. Bichet reports advisory board participation, honoraria and other financial support from Amicus Therapeutics, Inc. and Sanofi/Genzyme. Antonio Pisani declares no conflicts of interest. Albina Nowak reports advisory board, speaker honoraria and other financial support from Amicus Therapeutics, Inc., Sanofi Genzyme and Takeda. Roser Torra reports advisory board participation, consulting fees and honoraria for Amicus Therapeutics, Inc., Chiesi, Sanofi and Takeda. Aneal Khan reports research support from Amicus Therapeutics, Inc. Olga Azevedo reports financial support from Takeda, Amicus Therapeutics, Inc., Chiesi and Sanofi Genzyme. Anna Lehman reports research grants from Idorsia, Sanofi Genzyme and Amicus Therapeutics, Inc., consulting fees and advisory board honoraria from Takeda, Sanofi Genzyme and Amicus Therapeutics, Inc., and honoraria from Sanofi Genzyme. Aleš Linhart reports consulting fees, honoraria, advisory board membership and other financial support from Amicus Therapeutics, Inc., Chiesi, Sanofi and Takeda. Jasmine Rutecki and Joseph D. Giuliano are employees and shareholders of Amicus Therapeutics, Inc. Eva Krusinska reports working as a consultant under the contract of Pharmaland Consulting Group. Peter Nordbeck reports consulting and speaker fees from Abiomed, Amicus Therapeutics, Inc., Bayer, Boehringer Ingelheim, Boston Scientific, Cardiac Dimensions, Chiesi, Daiichi Sankyo, Idorsia, Sanofi/Genzyme and Shire/Takeda.
Figures


References
-
- Ortiz A, Germain DP, Desnick RJ, et al. Fabry disease revisited: management and treatment recommendations for adult patients. Mol Genet Metab. 2018;123:416‐427. - PubMed
-
- Rozenfeld P, Feriozzi S. Contribution of inflammatory pathways to Fabry disease pathogenesis. Mol Genet Metab. 2017;122:19‐27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

