Parkinson's Disease Drug Therapies in the Clinical Trial Pipeline: 2024 Update
- PMID: 39031388
- PMCID: PMC11307066
- DOI: 10.3233/JPD-240272
Parkinson's Disease Drug Therapies in the Clinical Trial Pipeline: 2024 Update
Abstract
Background: For the past five years, our annual reports have been tracking the clinical development of new drug-based therapies for the neurodegenerative condition of Parkinson's disease (PD). These reviews have followed the progress both of "symptomatic treatments" (ST - improves/reduces symptoms of the condition) and "disease-modifying treatments" (DMT - attempts to delay/slow progression by addressing the underlying biology of PD). Efforts have also been made to further categorize these experimental treatments based on their mechanisms of action and class of drug.
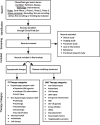
Methods: A dataset of clinical trials for drug therapies in PD using trial data downloaded from the ClinicalTrials.gov online registry was generated. A breakdown analysis of all the studies that were active as of January 31st, 2024, was conducted. This analysis involved categorizing the trials based on both the mechanism of action (MOA) and the drug target.
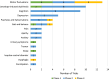
Results: There were 136 active Phase 1-3 trials evaluating drug therapies for PD registered on ClinicalTrials.gov, as of January 31, 2024. Of these trials, 76 (56%) were classified as ST trials and 60 (44%) were designated DMT. More than half (58%) of the trials were in Phase 2 testing stage, followed by Phase 1 (30%) and Phase 3 (12%). 35 of the trials were registered since our last report, with the remaining 101 trials appearing in at least one earlier report.
Conclusions: The drug development pipeline for PD remains in a robust state with a wide variety of approaches being developed and evaluated in Phase 1 and 2. Yet again, however, only a limited number of DMTs are transitioning to Phase 3.
Keywords: Clinical trials; Parkinson’s; disease modification; gene therapy; immunotherapy; inflammation; neuroprotection; studies.
Plain language summary
The development of new medical therapies, particularly for neurodegenerative conditions, is a long process that involves multiple phases of testing before a treatment is approved for use in a doctor’s clinic. The first phase assesses the short-term safety of a drug – most often in healthy volunteers but sometimes in people affected by the disease. The second phase explores the short-term safety and preliminary efficacy of the agent in people affected by the disease of interest, and the third phase investigates long-term safety and efficacy in a large group of people affected by the disease. For a disease like Parkinson’s disease, where the causes of the condition are not well understood, drugs targeting different biological pathways need to be tested to determine which ones may be useful in treating the symptoms, and which could be administered to slow down or stop the progression of the condition. Here, we provide an annual report on the current landscape of both these clinical testing efforts. In total, we reviewed 136 active studies evaluating therapies for Parkinson’s disease registered on a clinical trial database called ‘ClinicalTrials.gov’. Of these trials, approximately 55% were testing experimental symptomatic treatments, while the rest were focused on slowing down disease progression. More than half (58%) of the studies were in the second phase of clinical testing (short-term safety and preliminary efficacy), but only three studies were found to be testing treatments to stop the progression of Parkinson’s in the Phase 3 testing. We concluded that the drug development pipeline for Parkinson’s is robust, but more progress needs to be made with late-stage testing of treatments to slow the disease.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Vaxxinity Demonstrates Target Engagement of Toxic Alpha-Synuclein in Parkinson’s Patients, https://ir.vaxxinity.com/news-releases/news-release-details/vaxxinity-de... (2023, accessed 13 June 2024).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

