Design, construction and optimization of formaldehyde growth biosensors with broad application in biotechnology
- PMID: 39031508
- PMCID: PMC11259041
- DOI: 10.1111/1751-7915.14527
Design, construction and optimization of formaldehyde growth biosensors with broad application in biotechnology
Abstract
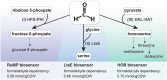
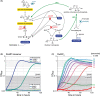
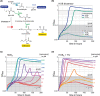
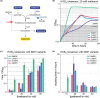
Formaldehyde is a key metabolite in natural and synthetic one-carbon metabolism. To facilitate the engineering of formaldehyde-producing enzymes, the development of sensitive, user-friendly, and cost-effective detection methods is required. In this study, we engineered Escherichia coli to serve as a cellular biosensor capable of detecting a broad range of formaldehyde concentrations. Using both natural and promiscuous formaldehyde assimilation enzymes, we designed three distinct E. coli growth biosensor strains that depend on formaldehyde for cell growth. These strains were engineered to be auxotrophic for one or several essential metabolites that could be produced through formaldehyde assimilation. The respective assimilating enzyme was expressed from the genome to compensate the auxotrophy in the presence of formaldehyde. We first predicted the formaldehyde dependency of the biosensors by flux balance analysis and then analysed it experimentally. Subsequent to strain engineering, we enhanced the formaldehyde sensitivity of two biosensors either through adaptive laboratory evolution or modifications at metabolic branch points. The final set of biosensors demonstrated the ability to detect formaldehyde concentrations ranging approximately from 30 μM to 13 mM. We demonstrated the application of the biosensors by assaying the in vivo activity of different methanol dehydrogenases in the most sensitive strain. The fully genomic nature of the biosensors allows them to be deployed as "plug-and-play" devices for high-throughput screenings of extensive enzyme libraries. The formaldehyde growth biosensors developed in this study hold significant promise for advancing the field of enzyme engineering, thereby supporting the establishment of a sustainable one-carbon bioeconomy.
© 2024 The Author(s). Microbial Biotechnology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Antoniewicz, M.R. (2019) Synthetic methylotrophy: strategies to assimilate methanol for growth and chemicals production. Current Opinion in Biotechnology, 59, 165–174. - PubMed
-
- Aslan, S. , Noor, E. , Benito Vaquerizo, S. , Lindner, S.N. & Bar‐Even, A. (2020) Design and engineering of E. Coli metabolic sensor strains with a wide sensitivity range for glycerate. Metabolic Engineering, 57, 96–109. - PubMed
-
- Bar‐Even, A. (2016) Formate assimilation: the metabolic architecture of natural and synthetic pathways. Biochemistry, 55, 3851–3863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

