KDM3A Ablation Activates Endogenous Retrovirus Expression to Stimulate Antitumor Immunity in Gastric Cancer
- PMID: 39031630
- PMCID: PMC11515915
- DOI: 10.1002/advs.202309983
KDM3A Ablation Activates Endogenous Retrovirus Expression to Stimulate Antitumor Immunity in Gastric Cancer
Abstract
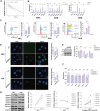
The success of immunotherapy for cancer treatment is limited by the presence of an immunosuppressive tumor microenvironment (TME); Therefore, identifying novel targets to that can reverse this immunosuppressive TME and enhance immunotherapy efficacy is essential. In this study, enrichment analysis based on publicly available single-cell and bulk RNA sequencing data from gastric cancer patients are conducted, and found that tumor-intrinsic interferon (IFN) plays a central role in TME regulation. The results shows that KDM3A over-expression suppresses the tumor-intrinsic IFN response and inhibits KDM3A, either genomically or pharmacologically, which effectively promotes IFN responses by activating endogenous retroviruses (ERVs). KDM3A ablation reconfigures the dsRNA-MAVS-IFN axis by modulating H3K4me2, enhancing the infiltration and function of CD8 T cells, and simultaneously reducing the presence of regulatory T cells, resulting in a reshaped TME in vivo. In addition, combining anti-PD1 therapy with KDM3A inhibition effectively inhibited tumor growth. In conclusions, this study highlights KDM3A as a potential target for TME remodeling and the enhancement of antitumor immunity in gastric cancer through the regulation of the ERV-MAVS-IFN axis.
Keywords: ERV; KDM3A; gastric cancer; immunotherapy.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Ca‐Cancer J. Clin. 2021, 71, 209. - PubMed
-
- a) Satoh T., Xu R. H., Chung H. C., Sun G. P., Doi T., Xu J. M., Tsuji A., Omuro Y., Li J., Wang J. W., Miwa H., Qin S. K., Chung I. J., Yeh K. H., Feng J. F., Mukaiyama A., Kobayashi M., Ohtsu A., Bang Y. J., J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2039; - PubMed
- b) Bang Y. J., Van Cutsem E., Feyereislova A., Chung H. C., Shen L., Sawaki A., Lordick F., Ohtsu A., Omuro Y., Satoh T., Aprile G., Kulikov E., Hill J., Lehle M., Rüschoff J., Kang Y. K., Lancet 2010, 376, 687. - PubMed
-
- Janjigian Y. Y., Shitara K., Moehler M., Garrido M., Salman P., Shen L., Wyrwicz L., Yamaguchi K., Skoczylas T., Campos Bragagnoli A., Liu T., Schenker M., Yanez P., Tehfe M., Kowalyszyn R., Karamouzis M. V., Bruges R., Zander T., Pazo‐Cid R., Hitre E., Feeney K., Cleary J. M., Poulart V., Cullen D., Lei M., Xiao H., Kondo K., Li M., Ajani J. A., Lancet 2021, 398, 27. - PMC - PubMed
-
- a) Lu Z., Yang S., Luo X., Shi Y., Lee J. S., Deva S., Liu T., Chao Y., Zhang Y., Huang R., Xu Y., Shen Z., Shen L., Gastric Cancer 2022, 25, 943. - PMC - PubMed
- b) Zhang P., Liu M., Cui Y., Zheng P., Liu Y., Brief. Bioinform.s 2021, 22, bbaa180;
- c) Le D. T., Uram J. N., Wang H., Bartlett B. R., Kemberling H., Eyring A. D., Skora A. D., Luber B. S., Azad N. S., Laheru D., Biedrzycki B., Donehower R. C., Zaheer A., Fisher G. A., Crocenzi T. S., Lee J. J., Duffy S. M., Goldberg R. M., de la Chapelle A., Koshiji M., Bhaijee F., Huebner T., Hruban R. H., Wood L. D., Cuka N., Pardoll D. M., Papadopoulos N., Kinzler K. W., Zhou S., Cornish T. C., et al., N. Engl. J. Med. 2015, 372, 2509. - PMC - PubMed
-
- a) Maio M., Ascierto P. A., Manzyuk L., Motola‐Kuba D., Penel N., Cassier P. A., Bariani G. M., De Jesus Acosta A., Doi T., Longo F., Miller W. H., Oh D. Y., Gottfried M., Xu L., Jin F., Norwood K., Marabelle A., Ann. Oncol. : Off. J. Eur. Soc. Med. Oncol. 2022, 33, 929; - PubMed
- b) Saeed A., Park R., Dai J., Al‐Rajabi R., Kasi A., Baranda J., Williamson S., Saeed A., Ripp J., Collins Z., Mulvaney K., Shugrue M., Firth‐Braun J., Godwin A. K., Madan R., Phadnis M., Sun W., Cell Rep. 2023, 4, 100916,; - PMC - PubMed
- c) Liu Y., Sethi N. S., Hinoue T., Schneider B. G., Cherniack A. D., Sanchez‐Vega F., Seoane J. A., Farshidfar F., Bowlby R., Islam M., Kim J., Chatila W., Akbani R., Kanchi R. S., Rabkin C. S., Willis J. E., Wang K. K., McCall S. J., Mishra L., Ojesina A. I., Bullman S., Pedamallu C. S., Lazar A. J., Sakai R., Thorsson V., Bass A. J., Laird P. W., Cancer Cell 2018, 33, 721; - PMC - PubMed
- d) Zhao W., Jia Y., Sun G., Yang H., Liu L., Qu X., Ding J., Yu H., Xu B., Zhao S., Xing L., Chai J., Nat. Commun. 2023, 14, 2985; - PMC - PubMed
- e) Kwon M., An M., Klempner S. J., Lee H., Kim K. M., Sa J. K., Cho H. J., Hong J. Y., Lee T., Min Y. W., Kim T. J., Min B. H., Park W. Y., Kang W. K., Kim K. T., Kim S. T., Lee J., Cancer Discov. 2021, 11, 2168; - PubMed
- f) Chen J., Liu K., Luo Y., Kang M., Wang J., Chen G., Qi J., Wu W., Wang B., Han Y., Shi L., Wang K., Han X., Ma X., Liu W., Ding Y., Wang L., Liang H., Wang L., Chen J., Gastroenterology 2023, 165, 88. - PubMed
MeSH terms
Substances
Grants and funding
- 82173829/National Natural Science Foundation of China
- 32370836/National Natural Science Foundation of China
- KY012023333/Excellent Young Talent Program of Guangdong Provincial People's Hospital
- B2022168/Medical Scientific Research Foundation of Guangdong Province of China
- NSZD2024023/Nanshan District health project
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
