Dose-dependent effect of megestrol acetate supplementation in cancer patients with anorexia-cachexia syndrome: A meta-analysis
- PMID: 39031821
- PMCID: PMC11294013
- DOI: 10.1002/jcsm.13500
Dose-dependent effect of megestrol acetate supplementation in cancer patients with anorexia-cachexia syndrome: A meta-analysis
Abstract
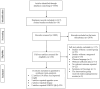
There is inconsistent evidence relating to the effects of megestrol acetate (MA) supplementation on cancer patients suffering from anorexia-cachexia syndrome. This review aimed to examine the dose-response effect of MA supplementation in patients with cancer-associated anorexia/cachexia. Relevant keywords were searched in PubMed, Scopus and ISI Web of Science from inception to June 2023 for randomized controlled trials (RCTs) examining the effect of MA on pathologies in patients with cancer-associated cachexia. Our primary outcomes were changes in body weight and appetite. However, fatigue and quality of life were secondary outcomes. The mean difference (MD) and 95% confidence interval (95% CI) were estimated using the random-effects method. Thirteen trials comprising 1229 participants (mean age 60 years) were identified. The results of our highest versus lowest analysis revealed that MA supplementation was not associated with any increase in body weight (MD: 0.64 kg, 95% CI [-0.11, 1.38], P = 0.093, I2 = 69.1%; GRADE = very low certainty). Twelve trials, including 14 effect sizes derived from 1369 patients (intervention = 689, control = 680), provided data on the effect of MA on body weight. Subgroup analyses showed a significant increase in body weight following short-term intervention (≤8 weeks) and a combination of radiation/chemotherapy as concurrent treatment. A linear dose-response meta-analysis indicated that each 200 mg/day increment in MA consumption had a significant increase in weight gain (MD: 0.44; 95% CI [0.13, 0.74], P = 0.005; I2 = 97.1%); however, the magnitude of the effect was small. MA administration significantly affected the quality of life based on pooled effect sizes (MD: 1.15, 95% CI [0.76, 1.54], P < 0.001, I2 = 0%; n = 2 RCTs including 176 patients; GRADE = very low certainty). However, no significant effect of MA supplementation was observed on appetite (MD: 0.29, 95% CI [-0.05, 0.64], P = 0.096, I2 = 18.3%; n = 3 RCTs including 163 patients; GRADE = very low certainty) and fatigue (MD: 0.14, 95% CI [-0.09, 0.36], P = 0.236, I2 = 0%; n = 2 RCTs including 300 patients; GRADE = very low certainty). With very low certainty of the evidence, MA supplementation may not lead to a significantly increased weight gain and other outcomes.
Keywords: anorexia; appetite; cachexia; cancer; megestrol acetate.
© 2024 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr 2017;36:49–64. - PubMed
-
- Esper DH, Harb WA. The cancer cachexia syndrome: a review of metabolic and clinical manifestations. Nutr Clin Pract 2005;20:369–376. - PubMed
-
- Mason MC, Garcia JM, Sansgiry S, Walder A, Berger DH, Anaya DA. Preoperative cancer cachexia and short‐term outcomes following surgery. J Surg Res 2016;205:398–406. - PubMed
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical