Toxoplasma gondii suppress human cord blood cell differentiation to the NK cell population
- PMID: 39031850
- PMCID: PMC11191221
- DOI: 10.1002/iid3.1329
Toxoplasma gondii suppress human cord blood cell differentiation to the NK cell population
Abstract
Background: Toxoplasma gondii is an obligate intracellular protozoan parasite that can invade all mammalian cells. It is well established that natural killer (NK) cells have critical protective roles in innate immunity during infections by intracellular pathogens. In the current study, we conducted an in vitro experiment to evaluate NK cell differentiation and activation from human umbilical cord blood mononuclear cells (UCB-MNCs) after infection with T. gondii tachyzoites.
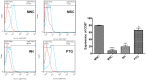
Methods: UCB-MNCs were infected by fresh tachyzoites of type I (RH) or type II (PTG) strains of T. gondii pre-expanded in mesenchymal stem cells for 2 weeks in a medium enriched with stem cell factor, Flt3, IL-2, and IL-15. Flow cytometry analysis and western blot analysis were performed to measure the CD57+, CD56+, and Granzyme A (GZMA).
Results: Data revealed that incubation of UCB-MNCs with NK cell differentiation medium increased the CD57+, CD56+, and GZMA. UCB-MNCs cocultured with PTG tachyzoites showed a significant reduction of CD56+ and GZMA, but nonsignificant changes, in the levels of CD56+ compared to the control UCB-MNCs (p > .05). Noteworthy, 2-week culture of UCB-MNCs with type I (RH) tachyzoites significantly suppressed CD57+, CD56+, and GZMA, showing reduction of NK cell differentiation from cord blood cells.
Conclusion: Our findings suggest that virulent T. gondii tachyzoites with cytopathic effects inhibit NK cell activation and eliminate innate immune responses during infection, and consequently enable the parasite to continue its survival in the host body.
Keywords: NK cells; Toxoplasma gondii; differentiation; functional activity; maturation; umbilical cord blood mononuclear cells.
© 2024 The Author(s). Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
[Effects of Toxoplasma gondii infection on mouse and human uterine natural killer cells].Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2022 Apr 7;34(2):149-157. doi: 10.16250/j.32.1374.2021272. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2022. PMID: 35537836 Chinese.
-
The role of IL-12 in stimulating NK cells against Toxoplasma gondii infection: a mini-review.Parasitol Res. 2021 Jul;120(7):2303-2309. doi: 10.1007/s00436-021-07204-w. Epub 2021 Jun 10. Parasitol Res. 2021. PMID: 34110502 Review.
-
Ex vivo expansion of T, NK and CD34+ cells from umbilical cord blood.Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005 Dec;13(6):1076-81. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005. PMID: 16403284
-
Ex vivo expansion of CD3depleted cord blood-MNCs in the presence of bone marrow stromal cells; an appropriate strategy to provide functional NK cells applicable for cellular therapy.Stem Cell Res. 2017 Mar;19:148-155. doi: 10.1016/j.scr.2017.01.010. Epub 2017 Feb 1. Stem Cell Res. 2017. PMID: 28171825
-
Innate immunity to Toxoplasma gondii infection.Nat Rev Immunol. 2014 Feb;14(2):109-21. doi: 10.1038/nri3598. Nat Rev Immunol. 2014. PMID: 24457485 Review.
Cited by
-
From pathogen to cure: exploring the antitumor potential of Toxoplasma gondii.Infect Agent Cancer. 2025 Jun 18;20(1):39. doi: 10.1186/s13027-025-00673-z. Infect Agent Cancer. 2025. PMID: 40533757 Free PMC article. Review.
-
Toxoplasma gondii modulates immune responses and mitigates type 1 diabetes progression in a streptozotocin-induced rat model.Cell Commun Signal. 2025 Apr 8;23(1):172. doi: 10.1186/s12964-025-02168-1. Cell Commun Signal. 2025. PMID: 40200271 Free PMC article.
-
Strain-dependent effects of Toxoplasma gondii on ovarian health and inflammation in a rat model.BMC Infect Dis. 2025 May 12;25(1):690. doi: 10.1186/s12879-025-11062-7. BMC Infect Dis. 2025. PMID: 40355816 Free PMC article.
References
-
- Safarpour H, Cevik M, Zarean M, et al. Global status of Toxoplasma gondii infection and associated risk factors in people living with HIV. AIDS. 2020;34(3):469‐474. - PubMed
-
- Yarovinsky F. Innate immunity to Toxoplasma gondii infection. Nat Rev Immunol. 2014;14(2):109‐121. - PubMed
-
- (a) Dubey JP. Advances in the life cycle of Toxoplasma gondii . Int J Parasitol. 1998;28(7):1019‐1024. - PubMed
-
- Harker KS. Toxoplasma gondii dissemination: a parasite's journey through the infected host. Parasite Immunol. 2015;37(3):141‐149. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

