BSA-stabilized selenium nanoparticles ameliorate intracerebral hemorrhage's-like pathology by inhibiting ferroptosis-mediated neurotoxicology via Nrf2/GPX4 axis activation
- PMID: 39032396
- PMCID: PMC11314897
- DOI: 10.1016/j.redox.2024.103268
BSA-stabilized selenium nanoparticles ameliorate intracerebral hemorrhage's-like pathology by inhibiting ferroptosis-mediated neurotoxicology via Nrf2/GPX4 axis activation
Abstract
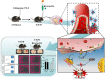
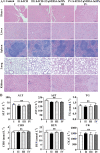
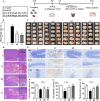
Intracerebral hemorrhage (ICH) is a prevalent hemorrhagic cerebrovascular emergency. Alleviating neurological damage in the early stages of ICH is critical for enhancing patient prognosis and survival rate. A novel form of cell death called ferroptosis is intimately linked to hemorrhage-induced brain tissue injury. Although studies have demonstrated the significant preventive impact of bovine serum albumin-stabilized selenium nanoparticles (BSA-SeNPs) against disorders connected to the neurological system, the neuroprotective effect on the hemorrhage stroke and the mechanism remain unknown. Therefore, based on the favorable biocompatibility of BSA-SeNPs, h-ICH (hippocampus-intracerebral hemorrhage) model was constructed to perform BSA-SeNPs therapy. As expected, these BSA-SeNPs could effectively improve the cognitive deficits and ameliorate the damage of hippocampal neuron. Furthermore, BSA-SeNPs reverse the morphology of mitochondria and enhanced the mitochondrial function, evidenced by mitochondrial respiration function (OCR) and mitochondrial membrane potential (MMP). Mechanistically, BSA-SeNPs could efficiently activate the Nrf2 to enhance the expression of antioxidant GPX4 at mRNA and protein levels, and further inhibit lipid peroxidation production in erastin-induced ferroptotic damages. Taken together, this study not only sheds light on the clinical application of BSA-SeNPs, but also provides its newly theoretical support for the strategy of the intervention and treatment of neurological impairment following ICH.
Keywords: BSA-selenium nanoparticles; Cognitive function; Ferroptosis; Intracerebral hemorrhage; Nrf2-GPX4 axis.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest All authors declare that there are no competing interests.
Figures






References
-
- Lim T.C., Mandeville E., Weng D., Wang L.S., Kurisawa M., Leite-Morris K., et al. Hydrogel-based therapy for brain repair after intracerebral hemorrhage. Translational stroke research. 2020;11:412–417. - PubMed
-
- Loan JJ, Kirby C, Emelianova K, Dando OR, Poon MT, Pimenova L, Hardingham GE, McColl BW, Klijn CJ, Al-Shahi Salman R, Schreuder FH, Samarasekera N. Secondary injury and inflammation after intracerebral haemorrhage: a systematic review and meta-analysis of molecular markers in patient brain tissue. J. Neurol. Neurosurg. Psychiatr. 2022 Feb;93(2):126–132. - PMC - PubMed
-
- Puy L, Parry-Jones AR, Sandset EC, Dowlatshahi D, Ziai W, Cordonnier C. Intracerebral haemorrhage. Nat. Rev. Dis. Primers. 2023 Mar 16;9(1):14. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

