Autoimmune inflammation triggers aberrant astrocytic calcium signaling to impair synaptic plasticity
- PMID: 39032542
- PMCID: PMC11415231
- DOI: 10.1016/j.bbi.2024.07.010
Autoimmune inflammation triggers aberrant astrocytic calcium signaling to impair synaptic plasticity
Abstract
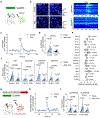
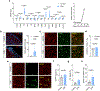
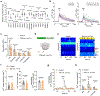
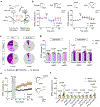
Cortical pathology involving inflammatory and neurodegenerative mechanisms is a hallmark of multiple sclerosis and a correlate of disease progression and cognitive decline. Astrocytes play a pivotal role in multiple sclerosis initiation and progression but astrocyte-neuronal network alterations contributing to gray matter pathology remain undefined. Here we unveil deregulation of astrocytic calcium signaling and astrocyte-to-neuron communication as key pathophysiological mechanisms of cortical dysfunction in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis. Using two-photon imaging ex vivo and fiber photometry in freely behaving mice, we found that acute EAE was associated with the emergence of spontaneously hyperactive cortical astrocytes exhibiting dysfunctional responses to cannabinoid, glutamate and purinoreceptor agonists. Abnormal astrocyte signaling by Gi and Gq protein coupled receptors was observed in the inflamed cortex. This was mirrored by treatments with pro-inflammatory factors both in vitro and ex vivo, suggesting cell-autonomous effects of the cortical neuroinflammatory environment. Finally, deregulated astrocyte calcium activity was associated with an enhancement of glutamatergic gliotransmission and a shift of astrocyte-mediated short-term and long-term plasticity mechanisms towards synaptic potentiation. Overall, our data identify astrocyte-neuronal network dysfunctions as key pathological features of gray matter inflammation in multiple sclerosis and potentially additional neuroimmunological disorders.
Keywords: Astrocyte; Calcium; Cortex; Gliotransmission; Multiple sclerosis.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

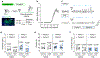
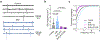
References
-
- Akerboom J, Carreras Calderón N, Tian L, Wabnig S, Prigge M, Tolö J, Gordus A, Orger MB, Severi KE, Macklin JJ, Patel R, Pulver SR, Wardill TJ, Fischer E, Schüler C, Chen TW, Sarkisyan KS, Marvin JS, Bargmann CI, Kim DS, Kügler S, Lagnado L, Hegemann P, Gottschalk A, Schreiter ER, Looger LL, 2013. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci 6, 2. 10.3389/fnmol.2013.00002. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

