HBcrAg values may predict virological and immunological responses to pegIFN-α in NUC-suppressed HBeAg-negative chronic hepatitis B
- PMID: 39033025
- PMCID: PMC11423235
- DOI: 10.1136/gutjnl-2024-332290
HBcrAg values may predict virological and immunological responses to pegIFN-α in NUC-suppressed HBeAg-negative chronic hepatitis B
Abstract
Objective: Selected populations of patients with chronic hepatitis B (CHB) may benefit from a combined use of pegylated interferon-alpha (pegIFN-α) and nucleos(t)ides (NUCs). The aim of our study was to assess the immunomodulatory effect of pegIFN-α on T and natural killer (NK) cell responses in NUC-suppressed patients to identify cellular and/or serological parameters to predict better T cell-restoring effect and better control of infection in response to pegIFN-α for a tailored application of IFN-α add-on.
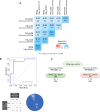
Design: 53 HBeAg-negative NUC-treated patients with CHB were randomised at a 1:1 ratio to receive pegIFN-α-2a for 48 weeks, or to continue NUC therapy and then followed up for at least 6 months maintaining NUCs. Serum hepatitis B surface antigen (HBsAg) and hepatitis B core-related antigen (HBcrAg) levels as well as peripheral blood NK cell phenotype and function and HBV-specific T cell responses upon in vitro stimulation with overlapping HBV peptides were measured longitudinally before, during and after pegIFN-α therapy.
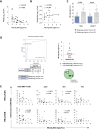
Results: Two cohorts of pegIFN-α treated patients were identified according to HBsAg decline greater or less than 0.5 log at week 24 post-treatment. PegIFN-α add-on did not significantly improve HBV-specific T cell responses during therapy but elicited a significant multispecific and polyfunctional T cell improvement at week 24 post-pegIFN-α treatment compared with baseline. This improvement was maximal in patients who had a higher drop in serum HBsAg levels and a lower basal HBcrAg values.
Conclusions: PegIFN-α treatment can induce greater functional T cell improvement and HBsAg decline in patients with lower baseline HBcrAg levels. Thus, HBcrAg may represent an easily and reliably applicable parameter to select patients who are more likely to achieve better response to pegIFN-α add-on to virally suppressed patients.
Keywords: Chronic HBV infection; HBcrAg; HBsAg; T cell functional reconstitution; immune modulatory treatments; pegylated IFN-a.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CF: Grant: Gilead, Abbvie. Consultant: Gilead, Abbvie, Vir Biotechnology Inc, Arrowhead, Transgene, BMS; MRB: speaker Bureau for AbbVie, Gilead, EISAI-MSD and advisor for AbbVie, Gilead, Janssen, AstraZeneca; PL: advisor and speaker bureau for Advisory Board/Speaker Bureau for Roche Pharma/diagnostics, Gilead Sciences, GSK, Abbvie, Janssen, Myr, Eiger, Antios, Aligos, Vir, Grifols, Altona, Roboscreen; SPF is employee of and stock-holder in Gilead Sciences, Inc. The remaining authors disclose no conflicts.
Figures





References
-
- Ghany MG, Buti M, Lampertico P, et al. Guidance on treatment endpoints and study design for clinical trials aiming to achieve cure in chronic hepatitis B and D: report from the 2022 AASLD-EASL HBV-HDV treatment endpoints conference. Hepatology. 2023;78:1654–73. doi: 10.1097/HEP.0000000000000431. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
