Computer-aided detection of tuberculosis from chest radiographs in a tuberculosis prevalence survey in South Africa: external validation and modelled impacts of commercially available artificial intelligence software
- PMID: 39033067
- PMCID: PMC11339183
- DOI: 10.1016/S2589-7500(24)00118-3
Computer-aided detection of tuberculosis from chest radiographs in a tuberculosis prevalence survey in South Africa: external validation and modelled impacts of commercially available artificial intelligence software
Erratum in
-
Correction to Lancet Digit Health 2024; 6: e605-13.Lancet Digit Health. 2024 Sep;6(9):e604. doi: 10.1016/S2589-7500(24)00176-6. Lancet Digit Health. 2024. PMID: 39179309 Free PMC article. No abstract available.
Abstract
Background: Computer-aided detection (CAD) can help identify people with active tuberculosis left undetected. However, few studies have compared the performance of commercially available CAD products for screening in high tuberculosis and high HIV settings, and there is poor understanding of threshold selection across products in different populations. We aimed to compare CAD products' performance, with further analyses on subgroup performance and threshold selection.
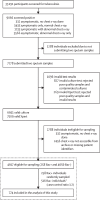
Methods: We evaluated 12 CAD products on a case-control sample of participants from a South African tuberculosis prevalence survey. Only those with microbiological test results were eligible. The primary outcome was comparing products' accuracy using the area under the receiver operating characteristic curve (AUC) against microbiological evidence. Threshold analyses were performed based on pre-defined criteria and across all thresholds. We conducted subgroup analyses including age, gender, HIV status, previous tuberculosis history, symptoms presence, and current smoking status.
Findings: Of the 774 people included, 516 were bacteriologically negative and 258 were bacteriologically positive. Diverse accuracy was noted: Lunit and Nexus had AUCs near 0·9, followed by qXR, JF CXR-2, InferRead, Xvision, and ChestEye (AUCs 0·8-0·9). XrayAME, RADIFY, and TiSepX-TB had AUC under 0·8. Thresholds varied notably across these products and different versions of the same products. Certain products (Lunit, Nexus, JF CXR-2, and qXR) maintained high sensitivity (>90%) across a wide threshold range while reducing the number of individuals requiring confirmatory diagnostic testing. All products generally performed worst in older individuals, people with previous tuberculosis, and people with HIV. Variations in thresholds, sensitivity, and specificity existed across groups and settings.
Interpretation: Several previously unevaluated products performed similarly to those evaluated by WHO. Thresholds differed across products and demographic subgroups. The rapid emergence of products and versions necessitates a global strategy to validate new versions and software to support CAD product and threshold selections.
Funding: Government of Canada.
Copyright © 2024 World Health Organization. Published by Elsevier Ltd. This is an Open Access article published under the CC BY 3.0 IGO license which permits users to download and share the article for non-commercial purposes, so long as the article is reproduced in the whole without changes, and provided the original source is properly cited. This article shall not be used or reproduced in association with the promotion of commercial products, services or any entity. There should be no suggestion that WHO endorses any specific organisation, products or services. The use of the WHO logo is not permitted. This notice should be preserved along with the article's original URL.
Conflict of interest statement
Declaration of interests CMD declares research grants from US National Institutes of Health, German Ministry of Education and Research, German Alliance for Global Health Research, United States Agency for International Development, FIND, German Center for Infection Research, and WHO. CMD also declares that she serves as an academic editor for PLoS Medicine and on the WHO Technical Advisory Group on tuberculosis diagnostics. All other authors declare no competing interests.
Figures

References
-
- WHO . World Health Organization; Geneva: 2023. Global tuberculosis report 2023.
-
- van Cleeff MRA, Kivihya-Ndugga L, Githui W, Nganga L, Odhiambo J, Klatser PR. A comprehensive study of the efficiency of the routine pulmonary tuberculosis diagnostic process in Nairobi. Int J Tuberc Lung Dis. 2003;7:186–189. - PubMed
-
- Pinto LM, Pai M, Dheda K, Schwartzman K, Menzies D, Steingart KR. Scoring systems using chest radiographic features for the diagnosis of pulmonary tuberculosis in adults: a systematic review. Eur Respir J. 2013;42:480–494. - PubMed
-
- Graham S, Das Gupta K, Hidvegi R J, et al. Chest radiograph abnormalities associated with tuberculosis: reproducibility and yield of active cases. Int J Tuberc Lung Dis. 2002;6:137–142. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

