Enhanced disulphide bond stability contributes to the once-weekly profile of insulin icodec
- PMID: 39033137
- PMCID: PMC11271312
- DOI: 10.1038/s41467-024-50477-9
Enhanced disulphide bond stability contributes to the once-weekly profile of insulin icodec
Abstract
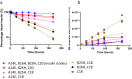
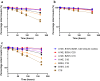
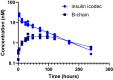
Insulin icodec is a once-weekly insulin analogue that has a long half-life of approximately 7 days, making it suitable for once weekly dosing. The Insulin icodec molecule was developed based on the hypothesis that lowering insulin receptor affinity and introducing a strong albumin-binding moiety would result in a long insulin half-life, provided that non-receptor-mediated clearance is diminished. Here, we report an insulin clearance mechanism, resulting in the splitting of insulin molecules into its A-chain and B-chain by a thiol-disulphide exchange reaction. Even though the substitutions in insulin icodec significantly stabilise insulin against such degradation, some free B-chain is observed in plasma samples from minipigs and people with type 2 diabetes. In summary, we identify thiol-disulphide exchange reactions to be an important insulin clearance mechanism and find that stabilising insulin icodec towards this reaction significantly contributes to its long pharmacokinetic/pharmacodynamic profile.
© 2024. The Author(s).
Conflict of interest statement
All authors are present employees of Novo Nordisk A/S and are shareholders of Novo Nordisk A/S.
Figures




References
-
- Muttenthaler, M., King, G. F., Adams, D. J. & Alewood, P. F. Trends in peptide drug discovery. Nat. Rev. Drug Discov.20, 309–325 (2021). - PubMed
-
- Rosenstock, J. & Del Prato, S. Basal weekly insulins: the way of the future! Metabolism126, 154924 (2022). - PubMed
-
- Kjeldsen, T. B. et al. Molecular engineering of insulin icodec, the first acylated insulin analog for once-weekly administration in humans. J. Med Chem.64, 8942–8950 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

