Extracellular vesicle surface display enhances the therapeutic efficacy and safety profile of cancer immunotherapy
- PMID: 39033322
- PMCID: PMC11489549
- DOI: 10.1016/j.ymthe.2024.07.013
Extracellular vesicle surface display enhances the therapeutic efficacy and safety profile of cancer immunotherapy
Abstract
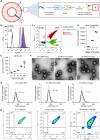
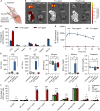
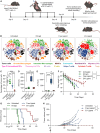
Immunotherapy has emerged as a mainstay in cancer therapy, yet its efficacy is constrained by the risk of immune-related adverse events. In this study, we present a nanoparticle-based delivery system that enhances the therapeutic efficacy of immunomodulatory ligands while concurrently limiting systemic toxicity. We demonstrate that extracellular vesicles (EVs), lipid bilayer enclosed particles released by cells, can be efficiently engineered via inverse electron demand Diels-Alder (iEDDA)-mediated conjugation to display multiple immunomodulatory ligands on their surface. Display of immunomodulatory ligands on the EV surface conferred substantial enhancements in signaling efficacy, particularly for tumor necrosis factor receptor superfamily (TNFRSF) agonists, where the EV surface display served as an alternative FcγR-independent approach to induce ligand multimerization and efficient receptor crosslinking. EVs displaying a complementary combination of immunotherapeutic ligands were able to shift the tumor immune milieu toward an anti-tumorigenic phenotype and significantly suppress tumor burden and increase survival in multiple models of metastatic cancer to a greater extent than an equivalent dose of free ligands. In summary, we present an EV-based delivery platform for cancer immunotherapeutic ligands that facilitates superior anti-tumor responses at significantly lower doses with fewer side effects than is possible with conventional delivery approaches.
Keywords: cancer immunotherapy; extracellular vesicles; iEDDA; nanomedicine; surface functionalization.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.T.N.L. is a scientific co-founder and advisor of Carmine Therapeutics, a start-up company that develops EV-based gene therapy.
Figures




References
-
- Lewis N.D., Sia C.L., Kirwin K., Haupt S., Mahimkar G., Zi T., Xu K., Dooley K., Jang S.C., Choi B., et al. Exosome Surface Display of IL12 Results in Tumor-Retained Pharmacology with Superior Potency and Limited Systemic Exposure Compared with Recombinant IL12. Mol. Cancer Ther. 2021;20:523–534. doi: 10.1158/1535-7163.MCT-20-0484. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

