A physiologically-based pharmacokinetic modeling approach for dosing amiodarone in children on ECMO
- PMID: 39033462
- PMCID: PMC11533098
- DOI: 10.1002/psp4.13199
A physiologically-based pharmacokinetic modeling approach for dosing amiodarone in children on ECMO
Abstract
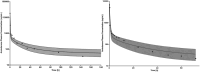
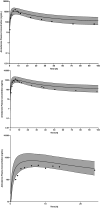
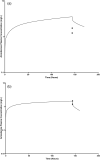
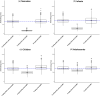
Extracorporeal membrane oxygenation (ECMO) is a cardiopulmonary bypass device commonly used to treat cardiac arrest in children. The American Heart Association guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care recommend using amiodarone as a first-line agent to treat ventricular arrhythmias in children with cardiac arrest. However, there are no dosing recommendations for amiodarone to treat ventricular arrhythmias in pediatric patients on ECMO. Amiodarone has a high propensity for adsorption to the ECMO components due to its physicochemical properties leading to altered pharmacokinetics (PK) in ECMO patients. The change in amiodarone PK due to interaction with ECMO components may result in a difference in optimal dosing in patients on ECMO when compared with non-ECMO patients. To address this clinical knowledge gap, a physiologically-based pharmacokinetic model of amiodarone was developed in adults and scaled to children, followed by the addition of an ECMO compartment. The pediatric model included ontogeny functions of cytochrome P450 (CYP450) enzyme maturation across various age groups. The ECMO compartment was parameterized using the adsorption data of amiodarone obtained from ex vivo studies. Model predictions captured observed concentrations of amiodarone in pediatric patients with ECMO well with an average fold error between 0.5 and 2. Model simulations support an amiodarone intravenous (i.v) bolus dose of 22 mg/kg (neonates), 13 mg/kg (infants), 8 mg/kg (children), and 6 mg/kg (adolescents). This PBPK modeling approach can be applied to explore the dosing of other drugs used in children on ECMO.
© 2024 The Author(s). CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
Similar articles
-
Physiologically Based Pharmacokinetic Approach to Determine Dosing on Extracorporeal Life Support: Fluconazole in Children on ECMO.CPT Pharmacometrics Syst Pharmacol. 2018 Oct;7(10):629-637. doi: 10.1002/psp4.12338. Epub 2018 Aug 13. CPT Pharmacometrics Syst Pharmacol. 2018. PMID: 30033691 Free PMC article.
-
Pharmacokinetics of intravenous amiodarone in children.Arch Dis Child. 2013 Dec;98(12):989-93. doi: 10.1136/archdischild-2013-304483. Epub 2013 Sep 19. Arch Dis Child. 2013. PMID: 24051016
-
Physiologically Based Pharmacokinetic Modelling in Critically Ill Children Receiving Anakinra While on Extracorporeal Life Support.Clin Pharmacokinet. 2024 Sep;63(9):1343-1356. doi: 10.1007/s40262-024-01424-w. Epub 2024 Sep 27. Clin Pharmacokinet. 2024. PMID: 39331235
-
Pediatric cardiovascular drug dosing in critically ill children and extracorporeal membrane oxygenation.J Cardiovasc Pharmacol. 2011 Aug;58(2):126-32. doi: 10.1097/FJC.0b013e318213aac2. J Cardiovasc Pharmacol. 2011. PMID: 21346597 Free PMC article. Review.
-
Pharmacokinetics and Dosing of Anti-infective Drugs in Patients on Extracorporeal Membrane Oxygenation: A Review of the Current Literature.Clin Ther. 2016 Sep;38(9):1976-94. doi: 10.1016/j.clinthera.2016.07.169. Epub 2016 Aug 21. Clin Ther. 2016. PMID: 27553752 Free PMC article. Review.
Cited by
-
Impact of Extracorporeal Membrane Oxygenation Life Support on Vancomycin Population Pharmacokinetics in Critical Illness: A Systematic Review.Clin Drug Investig. 2025 Aug 7. doi: 10.1007/s40261-025-01449-4. Online ahead of print. Clin Drug Investig. 2025. PMID: 40773131
References
-
- Rosario DC, Ambati S. Extracorporeal Membrane Oxygenation in Children. StatPearls; 2023. - PubMed
-
- Bembea MM, Ng DK, Rizkalla N, et al. Outcomes after extracorporeal cardiopulmonary resuscitation of pediatric in‐hospital cardiac arrest: a report from the get with the guidelines‐resuscitation and the extracorporeal life support organization registries. Crit Care Med. 2019;47:e278‐e285. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

