Impact of Race and Ethnicity on Outcomes After Umbilical Cord Blood Transplantation
- PMID: 39033978
- PMCID: PMC11842128
- DOI: 10.1016/j.jtct.2024.07.009
Impact of Race and Ethnicity on Outcomes After Umbilical Cord Blood Transplantation
Abstract
Background: Umbilical cord blood transplant (UCBT) improves access to transplant for patients lacking a fully matched donor. Previous Center for International Blood and Marrow Transplant Research (CIBMTR) showed that Black patients had a lower overall survival (OS) than White patients following single UCBT. The current study draws on a larger modern cohort and compares outcomes among White, Latinx, Black, and Asian patients.
Objective: To compare outcomes by social determinants of health.
Study design: We designed a retrospective study using CIBMTR data. US patients were between ages 1 and 80; 983 received single and 1529 double UCBT as reported to CIBMTR, following either a myeloablative (N = 1752) or reduced intensity conditioning (N = 759) for acute myeloid leukemia, acute lymphoid leukemia, or myelodysplasia. The primary outcome was 2-year OS. Secondary outcomes included disease free survival, transplant related mortality (TRM), acute and chronic graft vs host disease (GVHD), and GVHD free, relapse free survival (GRFS).
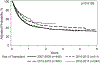
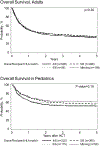
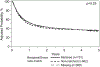
Results: For 1705 adults, in univariate analysis, 2-year OS was 41.5% (99% CI, 37.6 to 45.3) for Whites, 36.1% (99% CI, 28.2 to 44.5) for Latinx, 45.8% (99% CI, 36.7 to 55.1) for Blacks, and 44.5% (99% CI, 33.6 to 55.6) for Asians. In multivariate analysis of adults, Latinx patients had inferior OS compared to black patients (p = .0005, HR 1.45, 99% CI 1.18 to 1.79). OS improved over time for all racial/ethnic groups. GVHD rates were comparable among the different racial/ethnic groups. In the 807 children, the 2-year OS in univariate analysis was 66.1% (99% CI, 59.7 to 72.2) for Whites, 57.1% (99%CI, 49 to 64.9) for Latinx, 46.8% (99%CI, 35.3 to 58.4) for Blacks, and 53.8% (99%CI, 32.7 to 74.2) for Asians. In multivariate analysis, no difference in OS was observed among racial/ethnic groups (p = .051). Grade III/IV acute GVHD was higher in Blacks compared with Whites (p = .0016, HR 2.25, 99% CI 1.36 to 3.74) and Latinx (p = .0016, HR 2.17, 99% CI 1.43 to 3.30). There was no survival advantage to receiving a UCB unit from a donor of similar race and ethnicity, for any racial/ethnic groups, for both children and adults. Black and Latinx adult patients were more likely to live in areas defined as high poverty. Patients from high poverty level areas had worse OS (p = .03), due to a higher rate of TRM (p=0.04). Educational level, and type of insurance did not impact overall survival, GVHD, TRM or other transplant outcomes. Children from areas with a higher poverty level had higher TRM, regardless of race and ethnicity (p = .02). Public health insurance, such as Medicaid, was also associated with a higher TRM (p = .02). However, poverty did not impact pediatric OS, DFS, or other post-transplant outcomes.
Conclusions: OS for UCBT has improved over time. In adults, OS is comparable among Whites, Blacks, and Asians and lower for Latinx patients. In children, OS is comparable among Whites, Blacks, Latinx, and Asians, but Grade III/IV acute GVHD was higher in Black patients. There was no survival benefit to matching UCB unit and patient by race and ethnicity for adults and children.
Keywords: Cord Blood; Ethnicity; Race; Stem cell transplant.
Copyright © 2024 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

