Investigating the complex interplay between fibroblast activation protein α-positive cancer associated fibroblasts and the tumor microenvironment in the context of cancer immunotherapy
- PMID: 39035007
- PMCID: PMC11258004
- DOI: 10.3389/fimmu.2024.1352632
Investigating the complex interplay between fibroblast activation protein α-positive cancer associated fibroblasts and the tumor microenvironment in the context of cancer immunotherapy
Abstract
Introduction: This study investigates the role of Fibroblast Activation Protein (FAP)-positive cancer-associated fibroblasts (FAP+CAF) in shaping the tumor immune microenvironment, focusing on its association with immune cell functionality and cytokine expression patterns.
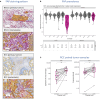
Methods: Utilizing immunohistochemistry, we observed elevated FAP+CAF density in metastatic versus primary renal cell carcinoma (RCC) tumors, with higher FAP+CAF correlating with increased T cell infiltration in RCC, a unique phenomenon illustrating the complex interplay between tumor progression, FAP+CAF density, and immune response.
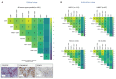
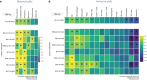
Results: Analysis of immune cell subsets in FAP+CAF-rich stromal areas further revealed significant correlations between FAP+ stroma and various T cell types, particularly in RCC and non-small cell lung cancer (NSCLC). This was complemented by transcriptomic analyses, expanding the range of stromal and immune cell subsets interrogated, as well as to additional tumor types. This enabled evaluating the association of these subsets with tumor infiltration, tumor vascularization and other components of the tumor microenvironment. Our comprehensive study also encompassed cytokine, angiogenesis, and inflammation gene signatures across different cancer types, revealing heterogeneous cellular composition, cytokine expressions and angiogenic profiles. Through cytokine pathway profiling, we explored the relationship between FAP+CAF density and immune cell states, uncovering potential immunosuppressive circuits that limit anti-tumor activity in tumor-resident immune cells.
Conclusions: These findings underscore the complexity of tumor biology and the necessity for personalized therapeutic and patient enrichment approaches. The insights gathered from FAP+CAF prevalence, immune infiltration, and gene signatures provide valuable perspectives on tumor microenvironments, aiding in future research and clinical strategy development.
Keywords: T cell infiltration; cancer immuno-therapy; fibroblast activation protein (FAP); immune cell subsets; patient enrichment; tumor immune microenvironment.
Copyright © 2024 Kraxner, Braun, Cheng, Yang, Pipaliya, Canamero, Andersson, Harring, Dziadek, Bröske, Ceppi, Tanos, Teichgräber and Charo.
Conflict of interest statement
Authors are paid employees and stockholders of Hoffman- LA Roche AG, Basel, Switzerland
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

