The protective effect of biotin supplementation and swimming training on cognitive impairment and mental symptoms in a rat model of Alzheimer's disease: A behavioral, biochemical, and histological study
- PMID: 39035497
- PMCID: PMC11259780
- DOI: 10.1016/j.heliyon.2024.e32299
The protective effect of biotin supplementation and swimming training on cognitive impairment and mental symptoms in a rat model of Alzheimer's disease: A behavioral, biochemical, and histological study
Abstract
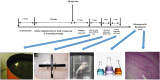
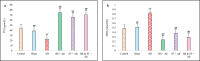
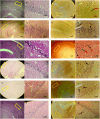
Vitamin B (Vit B) plays a regulatory role in cognitive memory and learning. We examined the biochemical and behavioral effects of biotin supplementation (BS) and swimming training (ST) on Alzheimer's disease (AD), the most common type of dementia, in male rats. Sixty rats were randomly assigned to six groups: control, sham (receiving phosphate-buffered saline), AD (receiving a single injection of Aβ into the lateral ventricle), ST (for 28 days and before Aβ injection), and BS (receiving BS through oral gavage for 28 days before Aβ injection). The treatments were continued until the end of the behavioral tests. Learning and memory functions were investigated through the Morris water maze (MWM) and depression and anxiety-like behaviors were tested by elevated plus-maze (EPM) and forced swimming tests. In addition, oxidative stress biomarkers, such as total thiol groups (TTG) and malondialdehyde (MDA) in serum were assessed and histological studies were performed using brain tissues. In the AD group, Aβ increased the distance traveled and escape latency in the MWM, but co-administration of BS and ST attenuated the results of the MWM, EPM, and FST tests. Furthermore, BS decreased the litigious biochemical effects of Aβ by enhancing the levels of TTG, in addition to reducing serum MDA levels. The use of BS as a potent antioxidant improved Aβ-induced memory impairment. It attenuated oxidative stress biomarkers in the brain (number of Aβ plaques) and serum of AD rats. We provide evidence for the use of BS in neurodegenerative disorders, such as AD, to elucidate the possible mechanisms.
Keywords: Alzheimer's disease; Aβ plaque; Biotin supplementation; Swimming training.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Alireza Komaki reports was provided by Hamadan University of Medical Sciences. Alireza Komaki reports a relationship with 10.13039/501100004697Hamadan University of Medical Sciences that includes: funding grants.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

