The Interaction of a Gemini Surfactant with a DNA Quadruplex
- PMID: 39035911
- PMCID: PMC11256301
- DOI: 10.1021/acsomega.4c03739
The Interaction of a Gemini Surfactant with a DNA Quadruplex
Abstract
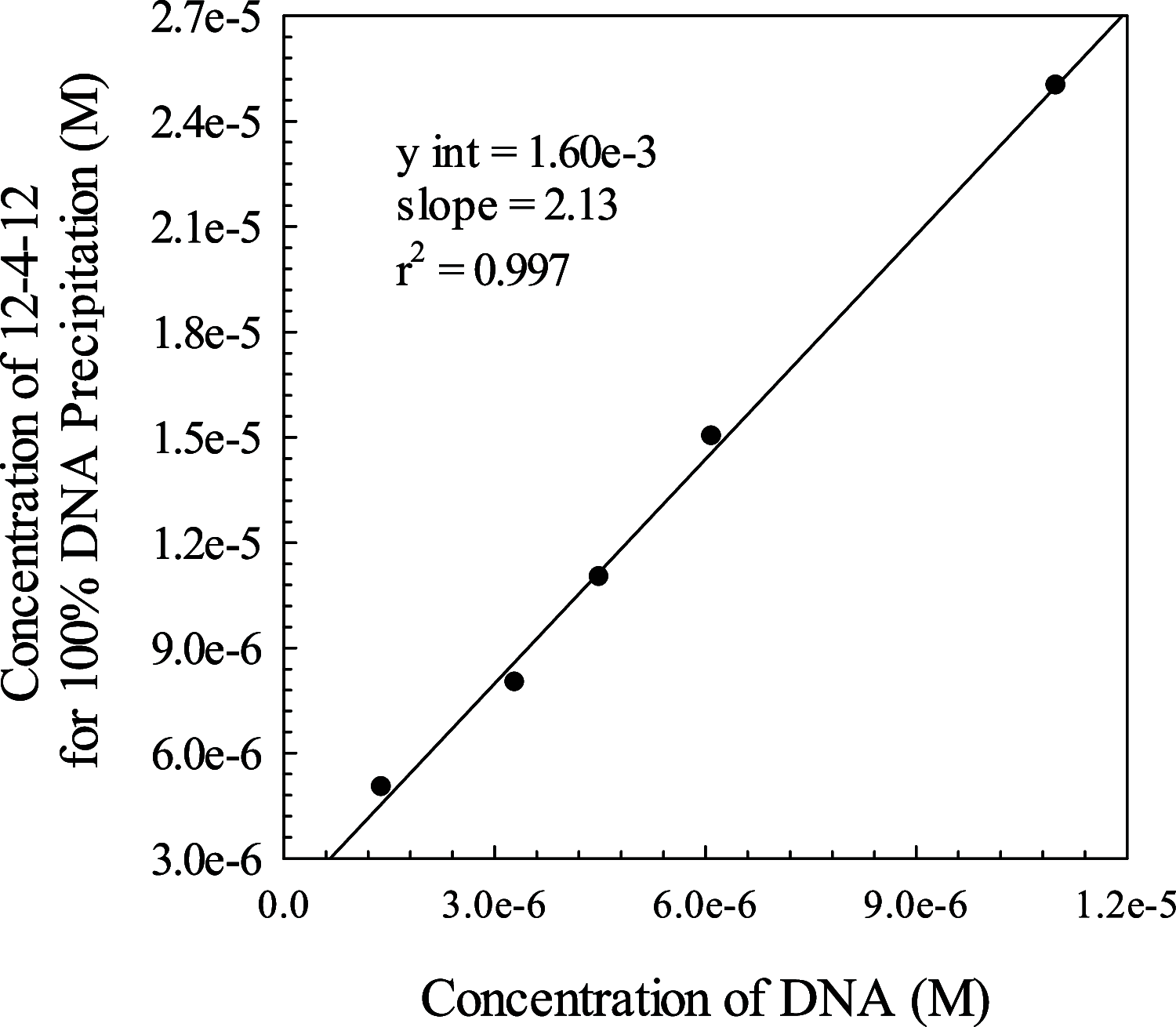
DNA secondary structures are stabilized by mono- and divalent cations. To examine the stability of the DNA quadruplex formed from (TTAGGG)4, its interaction with a dicationic Gemini surfactant in standard phosphate buffer was investigated. The Gemini surfactant begins to form micelles in buffer at a cmc (critical micelle concentration) of 1.5 mM. In this study, solutions of DNA were prepared in buffer with surfactant concentrations ranging from 0.0 to 3.0 mM, i.e., above and below the cmc of the surfactant. In all samples of DNA and surfactant, a precipitate formed. The fraction of DNA precipitated depends upon both the initial DNA concentration and the initial concentration of the surfactant. In those samples where the DNA did not totally precipitate, the residual DNA assumed a quadruplex conformation. It was determined that two surfactant molecules per DNA phosphate are needed to completely precipitate all of the DNA in a particular sample. An estimated apparent K sp for the DNA:surfactant complex was determined.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
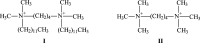





References
-
- Pal N.; Saxena N.; Mandel A. Studies on the physicochemical properties of synthesized tailor-made gemini surfactants for application in enhanced oil recovery. J. Mol. Liq. 2018, 258, 211–224. 10.1016/j.molliq.2018.03.037. - DOI
LinkOut - more resources
Full Text Sources
