Tolerability of a piezoelectric microneedle electroporator in human subjects
- PMID: 39036075
- PMCID: PMC11256137
- DOI: 10.1002/btm2.10662
Tolerability of a piezoelectric microneedle electroporator in human subjects
Abstract
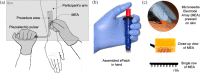
Electroporation, or the use of electric pulses to facilitate the intracellular delivery of DNA, RNA, and other molecules, is a well-established technique, that has been demonstrated to significantly augment the immunogenicity of DNA/mRNA vaccines and therapeutics. However, the clinical translation of traditional electroporators has been limited due to high costs, large size, complex user operation, and poor tolerability in humans due to nerve stimulation. In prior work, we introduced ePatch: an ultra-low-cost, handheld, battery-free electroporator employing a piezoelectric pulser coupled with a microneedle electrode array that showed enhanced immunogenic responses to an intradermal SARS-CoV-2 DNA vaccine in mice. The current study shifts focus from efficacy to tolerability, hypothesizing that ePatch's microneedle array, which localizes the electric field to the superficial skin strata, will minimize nerve stimulation and improve patient comfort. We tested this hypothesis in 14 healthy adults, monitoring pain and other potential adverse effects associated with electroporation. Compared to the insertion of a traditional hypodermic needle, the ePatch was less painful. Adverse effects such as pain, tenderness, erythema and swelling at the application sites were minimal, transient, and statistically indistinguishable between the experimental and placebo ePatch application, suggesting excellent tolerability towards electroporation. In summary, ePatch has a favorable tolerability profile in humans and offers the potential for the safe use of electroporation in a variety of clinical settings, including DNA and mRNA vaccination.
Keywords: DNA/RNA vaccination; electroporation; human skin tolerability; microneedle electrode array.
© 2024 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
MSB, GB and MRP are inventors of patents and co‐founders of Piezo Therapeutics, which is commercializing ePatch technology. Georgia Institute of Technology manages the associated conflicts of interest through its established procedures. The remaining authors declare no conflicts of interest.
Figures




References
-
- Zhang WW, Li L, Li D, et al. The first approved gene therapy product for cancer Ad‐p53 (Gendicine): 12 years in the clinic. Hum Gene Ther. 2018;29(2):160‐179. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

