Diffuse glioma molecular profiling with arterial spin labeling and dynamic susceptibility contrast perfusion MRI: A comparative study
- PMID: 39036439
- PMCID: PMC11259011
- DOI: 10.1093/noajnl/vdae113
Diffuse glioma molecular profiling with arterial spin labeling and dynamic susceptibility contrast perfusion MRI: A comparative study
Abstract
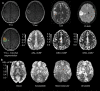
Background: Evaluation of molecular markers (IDH, pTERT, 1p/19q codeletion, and MGMT) in adult diffuse gliomas is crucial for accurate diagnosis and optimal treatment planning. Dynamic Susceptibility Contrast (DSC) and Arterial Spin Labeling (ASL) perfusion MRI techniques have both shown good performance in classifying molecular markers, however, their performance has not been compared side-by-side.
Methods: Pretreatment MRI data from 90 patients diagnosed with diffuse glioma (54 men/36 female, 53.1 ± 15.5 years, grades 2-4) were retrospectively analyzed. DSC-derived normalized cerebral blood flow/volume (nCBF/nCBV) and ASL-derived nCBF in tumor and perifocal edema were analyzed in patients with available IDH-mutation (n = 67), pTERT-mutation (n = 39), 1p/19q codeletion (n = 33), and MGMT promoter methylation (n = 31) status. Cross-validated uni- and multivariate logistic regression models assessed perfusion parameters' performance in molecular marker detection.
Results: ASL and DSC perfusion parameters in tumor and edema distinguished IDH-wildtype (wt) and pTERT-wt tumors from mutated ones. Univariate classification performance was comparable for ASL-nCBF and DSC-nCBV in IDH (maximum AUROCC 0.82 and 0.83, respectively) and pTERT (maximum AUROCC 0.70 and 0.81, respectively) status differentiation. The multivariate approach improved IDH (DSC-nCBV AUROCC 0.89) and pTERT (ASL-nCBF AUROCC 0.8 and DSC-nCBV AUROCC 0.86) classification. However, ASL and DSC parameters could not differentiate 1p/19q codeletion or MGMT promoter methylation status. Positive correlations were found between ASL-nCBF and DSC-nCBV/-nCBF in tumor and edema.
Conclusions: ASL is a viable gadolinium-free replacement for DSC for molecular characterization of adult diffuse gliomas.
Keywords: ASL; DSC; IDH; glioma; pTERT.
© The Author(s) 2024. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
There are no conflicts of interest to disclose.
Figures




References
-
- WHO Classification of Tumours Editorial Board. World Health Organization Classification of Tumours of the Central Nervous System. 5th ed. Lyon: International Agency for Research on Cancer; 2021.
-
- Crocetti E, Trama A, Stiller C, et al.; RARECARE Working Group. Epidemiology of glial and non-glial brain tumours in Europe. Eur J Cancer. 2012;48(10):1532–1542. - PubMed
-
- Brell M, Ibáñez J, Caral L, Ferrer E.. Factors influencing surgical complications of intra-axial brain tumours. Acta Neurochir (Wien). 2000;142(7):739–750. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
