Consensus on clinical diagnosis and medical treatment of HER2-low breast cancer (2022 edition)
- PMID: 39036662
- PMCID: PMC11256672
- DOI: 10.1016/j.jncc.2023.09.002
Consensus on clinical diagnosis and medical treatment of HER2-low breast cancer (2022 edition)
Abstract
Treatment of breast cancer with low expression of human epidermal growth factor receptor 2 (HER2; HER2-low) has drawn much attention in recent years. With the proven therapeutic effect of trastuzumab deruxtecan (T-DXd) in patients with HER2-low (immunohistochemistry [IHC] 1+, or IHC2+/in situ hybridization [ISH]-) breast cancer, HER2-low may become a new subtype of targeted therapy for breast cancer. The expert committee formulated this consensus based on the current clinical studies and clinical medication experience. The current consensus is the collaborative work of an interdisciplinary working group, including experts in the fields of pathology and oncology. The purpose of this consensus was to guide the clinical diagnosis and treatment of HER2-low breast cancer, thereby prolonging the overall survival of patients.
Keywords: Antibody-drug conjugate; Breast cancer; HER2-low.
© 2023 Chinese National Cancer Center. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures
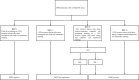
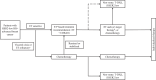

References
-
- Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28:1700–1712. doi: 10.1093/annonc/mdx308. - DOI - PMC - PubMed
-
- Ramakrishna N, Temin S, Chandarlapaty S, et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO clinical practice guideline update. J Clin Oncol. 2018;36:2804–2807. doi: 10.1200/JCO.2018.79.2713. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

