Trace amine-associated receptor 1 (TAAR1) agonism for psychosis: a living systematic review and meta-analysis of human and non-human data
- PMID: 39036710
- PMCID: PMC11258611
- DOI: 10.12688/wellcomeopenres.21302.1
Trace amine-associated receptor 1 (TAAR1) agonism for psychosis: a living systematic review and meta-analysis of human and non-human data
Abstract
Background: Trace amine-associated receptor 1 (TAAR1) agonism shows promise for treating psychosis, prompting us to synthesise data from human and non-human studies.
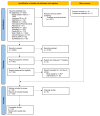
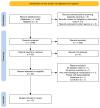
Methods: We co-produced a living systematic review of controlled studies examining TAAR1 agonists in individuals (with or without psychosis/schizophrenia) and relevant animal models. Two independent reviewers identified studies in multiple electronic databases (until 17.11.2023), extracted data, and assessed risk of bias. Primary outcomes were standardised mean differences (SMD) for overall symptoms in human studies and hyperlocomotion in animal models. We also examined adverse events and neurotransmitter signalling. We synthesised data with random-effects meta-analyses.
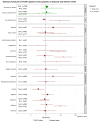
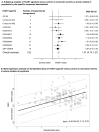
Results: Nine randomised trials provided data for two TAAR1 agonists (ulotaront and ralmitaront), and 15 animal studies for 10 TAAR1 agonists. Ulotaront and ralmitaront demonstrated few differences compared to placebo in improving overall symptoms in adults with acute schizophrenia (N=4 studies, n=1291 participants; SMD=0.15, 95%CI: -0.05, 0.34), and ralmitaront was less efficacious than risperidone (N=1, n=156, SMD=-0.53, 95%CI: -0.86, -0.20). Large placebo response was observed in ulotaront phase-III trials. Limited evidence suggested a relatively benign side-effect profile for TAAR1 agonists, although nausea and sedation were common after a single dose of ulotaront. In animal studies, TAAR1 agonists improved hyperlocomotion compared to control (N=13 studies, k=41 experiments, SMD=1.01, 95%CI: 0.74, 1.27), but seemed less efficacious compared to dopamine D 2 receptor antagonists (N=4, k=7, SMD=-0.62, 95%CI: -1.32, 0.08). Limited human and animal data indicated that TAAR1 agonists may regulate presynaptic dopaminergic signalling.
Conclusions: TAAR1 agonists may be less efficacious than dopamine D 2 receptor antagonists already licensed for schizophrenia. The results are preliminary due to the limited number of drugs examined, lack of longer-term data, publication bias, and assay sensitivity concerns in trials associated with large placebo response. Considering their unique mechanism of action, relatively benign side-effect profile and ongoing drug development, further research is warranted.
Registration: PROSPERO-ID: CRD42023451628.
Keywords: Antipsychotics; TAAR1; clinical trials; living evidence; meta-analysis; preclinical studies; schizophrenia; systematic review.
Plain language summary
There is a need for more effective treatments for psychosis, including schizophrenia. Psychosis is a collection of mental health symptoms, such as hearing voices, that can cause distress and impair functioning. These symptoms are thought to be caused by changes in a chemical messenger system in the brain called dopamine. Currently used antipsychotic medications target brain receptors that respond to dopamine. They are not effective in some people and can cause uncomfortable adverse events, such as weight gain and movement disorders, especially with long-term use. A new type of drug is the trace amine-associated receptor 1 (TAAR1) agonists. These drugs act on different brain receptors that can affect the activity of the dopamine system, but do not directly bind to dopamine receptors. We aimed to understand if TAAR1 agonists can reduce symptoms of psychosis, what adverse events they might have, and how they work. We did this by reviewing and collating all available evidence until November 2023. This is a “living” systematic review, so it will be regularly updated in the future. We looked at both human and animal studies investigating TAAR1 agonists. Human studies suggested that two TAAR1 agonists (namely, ulotaront or ralmitaront) might have little to no effect on reducing symptoms of psychosis compared to placebo in people with schizophrenia. They seemed to cause fewer adverse events than current antipsychotics. Data from animal studies suggested that TAAR1 agonists had some positive effects but potentially smaller than other antipsychotics. There were little to no data from both human and animal studies about how TAAR1 agonists actually work. From the current evidence we are uncertain about these results. With the ongoing development of new TAAR1 agonists, more evidence is needed to understand their potential role in the treatment of psychosis.
Copyright: © 2024 Siafis S et al.
Conflict of interest statement
Competing interests: Andrea Cipriani received research, educational and consultancy fees from the Italian Network for Paediatric Trials, CARIPLO Foundation, Lundbeck, and Angelini Pharma. Toshi A. Furukawa reports personal fees from Boehringer-Ingelheim, Daiichi Sankyo, DT Axis, Kyoto University Original, Shionogi, and SONY, and a grant from Shionogi outside the submitted work; in addition, TAF has patents 2020-548587 and 2022-082495 pending, and intellectual properties for Kokoro-app licensed to Mitsubishi-Tanabe. Oliver D. Howes has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organised by Angellini, Autifony, Biogen, Boehringer-Ingelheim, Eli Lilly, Elysium, Heptares, Global Medical Education, Invicro, Jansenn, Karuna, Lundbeck, Merck, Neurocrine, Ontrack/ Pangea, Otsuka, Sunovion, Recordati, Roche, Rovi and Viatris/ Mylan. He was previously a part-time employee of Lundbeck A/v. Dr Howes has a patent for the use of dopaminergic imaging. In the last three years Stefan Leucht has received honoraria for advising/consulting and/or for lectures and/or for educational material from Angelini, Boehringer Ingelheim, Eisai, Ekademia, GedeonRichter, Janssen, Karuna, Kynexis, Lundbeck, Medichem, Medscape, Mitsubishi, Otsuka, NovoNordisk, Recordati, Rovi, Teva. Robert A. McCutcheon has received speaker/consultancy fees from Karuna, Janssen, Boehringer Ingelheim, and Otsuka, and is a director of a company that hosts psychotropic prescribing decision tools. Carmen Moreno received honoraria as a consultant and/or advisor and/or for lectures from Angelini, Compass, Esteve, Exeltis Janssen, Lundbeck, Neuraxpharm, Nuvelution, Otsuka, Pfizer, Servier and Sunovion outside the submitted work. Nobuyuki Nomura has received speaker fees from Eisai, Meiji Seika Pharma, Otsuka, and Sumitomo Pharma; and manuscript fees from Sumitomo Pharma. Edoardo G. Ostinelli has received research and consultancy fees from Angelini Pharma. No other competing interests were disclosed.
Figures


References
-
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. : Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–51. 10.1016/S0140-6736(19)31135-3 - DOI - PMC - PubMed
-
- Schneider-Thoma J, Chalkou K, Dörries C, et al. : Comparative efficacy and tolerability of 32 oral and long-acting injectable antipsychotics for the maintenance treatment of adults with schizophrenia: a systematic review and network meta-analysis. Lancet. 2022;399(10327):824–36. 10.1016/S0140-6736(21)01997-8 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

