Recording from Snail Motor Nerves to Investigate Central Pattern Generation
- PMID: 39036715
- PMCID: PMC11256379
- DOI: 10.59390/JCRT2250
Recording from Snail Motor Nerves to Investigate Central Pattern Generation
Abstract
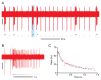
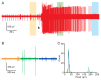
Feeding in pond snails has long been a model system for central pattern generation and its modulation. The pattern is generated by a small set of neurons in the buccal ganglia, which innervate the buccal mass, esophagus, and salivary glands. In this exercise, students observe feeding behavior and then record and quantify rhythmic motor activity and its response to feeding stimulants and neuromodulators. In a standard three-hour class period, students do a dissection, record from several nerves, and perform experimental manipulations such as adding feeding stimulants, serotonin, or dopamine to the preparation. Depending on the course goals, data can be presented qualitatively or cyclic measurements and spike-rate analysis can be done. This exercise leads to discussion of neural circuitry and intrinsic properties that support pattern generation for rhythmic activities such as feeding, locomotion, and respiration.
Keywords: CPG; Helisoma; Lymnaea; Planorbis; buccal; dopamine; extracellular; feeding; neuromodulation; pond snail; serotonin.
Copyright © 2022 Faculty for Undergraduate Neuroscience.
Figures









Similar articles
-
Plasticity in the multifunctional buccal central pattern generator of Helisoma illuminated by the identification of phase 3 interneurons.J Neurophysiol. 1996 Feb;75(2):561-74. doi: 10.1152/jn.1996.75.2.561. J Neurophysiol. 1996. PMID: 8714635
-
Control of feeding movements in the freshwater snail Planorbis corneus. I. Rhythmical neurons of buccal ganglia.Exp Brain Res. 1988;70(2):310-22. doi: 10.1007/BF00248356. Exp Brain Res. 1988. PMID: 3384034
-
Histochemical study on the relation between NO-generative neurons and central circuitry for feeding in the pond snail, Lymnaea stagnalis.Neurosci Res. 1998 Sep;32(1):57-63. doi: 10.1016/s0168-0102(98)00066-2. Neurosci Res. 1998. PMID: 9831252
-
The neuronal basis of feeding in the snail, Helisoma, with comparisons to selected gastropods.Prog Neurobiol. 2001 Mar;63(4):383-408. doi: 10.1016/s0301-0082(00)00049-6. Prog Neurobiol. 2001. PMID: 11163684 Review.
-
A systems approach to the cellular analysis of associative learning in the pond snail Lymnaea.Learn Mem. 2000 May-Jun;7(3):124-31. doi: 10.1101/lm.7.3.124. Learn Mem. 2000. PMID: 10837501 Review.
References
-
- Benjamin PR. Lymnaea . Scholarpedia. 2008;3:4124.
-
- Booth JRH, Sane V, Gather MC, Pulver SR. Inexpensive methods for live imaging of central pattern generator activity in the Drosophila larval motor system. J Undergrad Neurosci Educ. 2020;19:A124–A133. Available at https://pubmed.ncbi.nlm.nih.gov/33880100/ - PMC - PubMed
-
- Dillon RT colleagues. The Freshwater Gastropods of North America Volume 1: Atlantic drainages, Georgia through Pennsylvania. Charleston, SC: FWGNA Press; 2019. Available at https://www.fwgna.org/
LinkOut - more resources
Full Text Sources
Research Materials
