Adaptive Image Segmentation Reveals Substantial Cortical Bone Remodeling During Early Fracture Repair
- PMID: 39036745
- PMCID: PMC11257215
- DOI: 10.1080/21681163.2024.2345165
Adaptive Image Segmentation Reveals Substantial Cortical Bone Remodeling During Early Fracture Repair
Abstract
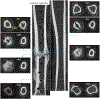
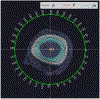
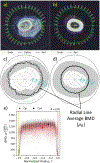
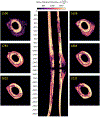
The goal of this study was to develop an image analysis algorithm for quantifying the effects of remodeling on cortical bone during early fracture healing. An adaptive thresholding technique with boundary curvature and tortuosity control was developed to automatically identify the endocortical and pericortical boundaries in the presence of high-gradient bone mineral density (BMD) near the healing zone. The algorithm successfully segmented more than 47,000 microCT images from 12 healing ovine osteotomies and intact contralateral tibiae. Resampling techniques were used to achieve data dimensionality reduction on the segmented images, allowing characterization of radial and axial distributions of cortical BMD. Local (transverse slice) and total (whole bone) remodeling scores were produced. These surrogate measures of cortical remodeling derived from BMD revealed that cortical changes were detectable throughout the region covered by callus and that the localized loss of cortical BMD was highest near the osteotomy. Total remodeling score was moderately and significantly correlated with callus volume and mineral composition (r > 0.64, p < 0.05), suggesting that the cortex may be a source of mineral needed to build callus.
Keywords: adaptive thresholding; bone fracture healing; cortical remodeling; micro-computed tomography; ovine osteotomy.
Conflict of interest statement
Declaration of Interest Statement The authors have no conflicts of interest relevant to this work.
Figures





Similar articles
-
Image-based radiodensity profilometry measures early remodeling at the bone-callus interface in sheep.Biomech Model Mechanobiol. 2022 Apr;21(2):615-626. doi: 10.1007/s10237-021-01553-2. Epub 2022 Jan 8. Biomech Model Mechanobiol. 2022. PMID: 34997398
-
Far cortical locking can improve healing of fractures stabilized with locking plates.J Bone Joint Surg Am. 2010 Jul 7;92(7):1652-60. doi: 10.2106/JBJS.I.01111. J Bone Joint Surg Am. 2010. PMID: 20595573 Free PMC article.
-
Increased cortical remodeling after osteotomy causes posttraumatic osteopenia.Bone. 2008 Sep;43(3):539-43. doi: 10.1016/j.bone.2008.05.017. Epub 2008 Jun 30. Bone. 2008. PMID: 18586599
-
The Impact of Strontium Ranelate on Metaphyseal Bone Healing in Ovariectomized Rats.Calcif Tissue Int. 2015 Oct;97(4):391-401. doi: 10.1007/s00223-015-0019-0. Epub 2015 Jun 18. Calcif Tissue Int. 2015. PMID: 26084691
-
Is a change in bone mineral density a sensitive and specific surrogate of anti-fracture efficacy?Bone. 2007 Sep;41(3):308-17. doi: 10.1016/j.bone.2007.06.010. Epub 2007 Jun 26. Bone. 2007. PMID: 17644058 Review.
Cited by
-
Don't mind the gap: reframing the Perren strain rule for fracture healing using insights from virtual mechanical testing.Bone Joint Res. 2025 Jan 1;14(1):5-15. doi: 10.1302/2046-3758.141.BJR-2024-0191.R2. Bone Joint Res. 2025. PMID: 39740681 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
