Loss-of-function in RBBP5 results in a syndromic neurodevelopmental disorder associated with microcephaly
- PMID: 39036895
- PMCID: PMC11648989
- DOI: 10.1016/j.gim.2024.101218
Loss-of-function in RBBP5 results in a syndromic neurodevelopmental disorder associated with microcephaly
Abstract
Purpose: Epigenetic dysregulation has been associated with many inherited disorders. RBBP5 (HGNC:9888) encodes a core member of the protein complex that methylates histone 3 lysine-4 and has not been implicated in human disease.
Methods: We identify 5 unrelated individuals with de novo heterozygous variants in RBBP5. Three nonsense/frameshift and 2 missense variants were identified in probands with neurodevelopmental symptoms, including global developmental delay, intellectual disability, microcephaly, and short stature. Here, we investigate the pathogenicity of the variants through protein structural analysis and transgenic Drosophila models.
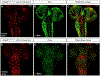
Results: Both missense p.(T232I) and p.(E296D) variants affect evolutionarily conserved amino acids located at the interface between RBBP5 and the nucleosome. In Drosophila, overexpression analysis identifies partial loss-of-function mechanisms when the variants are expressed using the fly Rbbp5 or human RBBP5 cDNA. Loss of Rbbp5 leads to a reduction in brain size. The human reference or variant transgenes fail to rescue this loss and expression of either missense variant in an Rbbp5 null background results in a less severe microcephaly phenotype than the human reference, indicating both missense variants are partial loss-of-function alleles.
Conclusion: Haploinsufficiency of RBBP5 observed through de novo null and hypomorphic loss-of-function variants is associated with a syndromic neurodevelopmental disorder.
Keywords: Epigenetic; H3K4 methylation; Microcephaly; Neurodevelopmental disorder; RBBP5.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing conducted at Baylor Genetics Laboratories.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

