The human mitochondrial translation factor TACO1 alleviates mitoribosome stalling at polyproline stretches
- PMID: 39036954
- PMCID: PMC11381339
- DOI: 10.1093/nar/gkae645
The human mitochondrial translation factor TACO1 alleviates mitoribosome stalling at polyproline stretches
Abstract
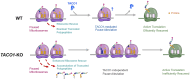
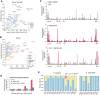
The prokaryotic translation elongation factor P (EF-P) and the eukaryotic/archaeal counterparts eIF5A/aIF5A are proteins that serve a crucial role in mitigating ribosomal stalling during the translation of specific sequences, notably those containing consecutive proline residues (1,2). Although mitochondrial DNA-encoded proteins synthesized by mitochondrial ribosomes also contain polyproline stretches, an EF-P/eIF5A mitochondrial counterpart remains unidentified. Here, we show that the missing factor is TACO1, a protein causative of a juvenile form of neurodegenerative Leigh's syndrome associated with cytochrome c oxidase deficiency, until now believed to be a translational activator of COX1 mRNA. By using a combination of metabolic labeling, puromycin release and mitoribosome profiling experiments, we show that TACO1 is required for the rapid synthesis of the polyproline-rich COX1 and COX3 cytochrome c oxidase subunits, while its requirement is negligible for other mitochondrial DNA-encoded proteins. In agreement with a role in translation efficiency regulation, we show that TACO1 cooperates with the N-terminal extension of the large ribosomal subunit bL27m to provide stability to the peptidyl-transferase center during elongation. This study illuminates the translation elongation dynamics within human mitochondria, a TACO1-mediated biological mechanism in place to mitigate mitoribosome stalling at polyproline stretches during protein synthesis, and the pathological implications of its malfunction.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Doerfel L.K., Wohlgemuth I., Kothe C., Peske F., Urlaub H., Rodnina M.V.. EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science. 2013; 339:85–88. - PubMed
-
- Rackham O., Filipovska A.. Organization and expression of the mammalian mitochondrial genome. Nat. Rev. Genet. 2022; 23:606–623. - PubMed
-
- Khawaja A., Cipullo M., Krüger A., Rorbach J.. Insights into mitoribosomal biogenesis from recent structural studies. Trends Biochem. Sci. 2023; 48:629–641. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

