SARS-CoV-2 Monoclonal Antibody Treatment Followed by Vaccination Shifts Human Memory B-Cell Epitope Recognition, Suggesting Antibody Feedback
- PMID: 39036987
- PMCID: PMC11566236
- DOI: 10.1093/infdis/jiae371
SARS-CoV-2 Monoclonal Antibody Treatment Followed by Vaccination Shifts Human Memory B-Cell Epitope Recognition, Suggesting Antibody Feedback
Abstract
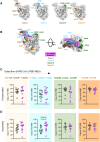
Therapeutic monoclonal antibodies (mAbs) have been studied in humans, but the impact on immune memory of mAb treatment during an ongoing infection remains unclear. We evaluated the effect of infusion of the anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike receptor-binding domain (RBD) mAb bamlanivimab on memory B cells (MBCs) in SARS-CoV-2-infected individuals. Bamlanivimab treatment skewed the repertoire of MBCs targeting spike toward non-RBD epitopes. Furthermore, the relative affinity of RBD MBCs was weaker in mAb-treated individuals compared to placebo-treated individuals over time. Subsequently, after mRNA coronavirus disease 2019 vaccination, MBC differences persisted and mapped to a specific reduction in recognition of the class II RBD site, the same RBD epitope recognized by bamlanivimab. These findings indicate a substantial role of antibody feedback in regulating MBC responses to infection, and single mAb administration can continue to impact MBC responses to additional antigen exposures months later.
Keywords: COVID-19; SARS-CoV-2; bamlanivimab; mAb therapy; memory B cells.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. S. C. has consulted for GSK, JP Morgan, Citi, Morgan Stanley, Avalia NZ, Nutcracker Therapeutics, University of California, California State Universities, United Airlines, Adagio, Sanofi, and Roche. J. S. C. has consulted for Merck and Company. P. K. is an employee and shareholder of Eli Lilly and Company. K. W. C. has consulted for Pardes Biosciences. D. M. S. has consulted for and has equity stake in Linear Therapies, Model Medicines, and Vx Biosciences and has consulted for Bayer, Kiadis, Signant Health, and Brio Clinical. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



Update of
-
SARS-CoV-2 monoclonal antibody treatment followed by vaccination shifts human memory B cell epitope recognition suggesting antibody feedback.bioRxiv [Preprint]. 2023 Nov 22:2023.11.21.567575. doi: 10.1101/2023.11.21.567575. bioRxiv. 2023. Update in: J Infect Dis. 2024 Nov 15;230(5):1187-1196. doi: 10.1093/infdis/jiae371. PMID: 38045374 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- UM1 AI069481/AI/NIAID NIH HHS/United States
- AI142742/Collaborative Center for Human Immunology
- U01 AI068636/AI/NIAID NIH HHS/United States
- 75N93019C00065/AI/NIAID NIH HHS/United States
- U54 CA267776/CA/NCI NIH HHS/United States
- A. P. Giannini Foundation
- U54CA267776/NIH Faculty Institutional Recruitment for Sustainable Transformation
- National Institute of Allergy and Infectious Diseases
- T32 AI007036/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- NH/NIH HHS/United States
- 75N93019C00065/GF/NIH HHS/United States
- La Jolla Institute for Immunology institutional funds
- U01 AI069424/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- U19 AI142742/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

