Thymic epithelial organoids mediate T-cell development
- PMID: 39036995
- PMCID: PMC11441983
- DOI: 10.1242/dev.202853
Thymic epithelial organoids mediate T-cell development
Abstract
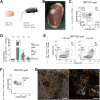
Although the advent of organoids has opened unprecedented perspectives for basic and translational research, immune system-related organoids remain largely underdeveloped. Here, we established organoids from the thymus, the lymphoid organ responsible for T-cell development. We identified conditions enabling mouse thymic epithelial progenitor cell proliferation and development into organoids with diverse cell populations and transcriptional profiles resembling in vivo thymic epithelial cells (TECs) more closely than traditional TEC cultures. In contrast to these two-dimensional cultures, thymic epithelial organoids maintained thymus functionality in vitro and mediated physiological T-cell development upon reaggregation with T-cell progenitors. The reaggregates showed in vivo-like epithelial diversity and the ability to attract T-cell progenitors. Thymic epithelial organoids are the first organoids originating from the stromal compartment of a lymphoid organ. They provide new opportunities to study TEC biology and T-cell development in vitro, paving the way for future thymic regeneration strategies in ageing or acute injuries.
Keywords: Mouse; Organoids; T cells; Thymic epithelial cells; Thymopoiesis; Thymus.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests M.P.L. is an employee of F. Hoffmann-La Roche.
Figures



References
-
- Asnaghi, M. A., Barthlott, T., Gullotta, F., Strusi, V., Amovilli, A., Hafen, K., Srivastava, G., Oertle, P., Toni, R., Wendt, D.et al. (2021). Thymus extracellular matrix–derived scaffolds support graft–resident thymopoiesis and long–term in vitro culture of adult thymic epithelial cells. Adv. Funct. Mater. 31, 2010747. 10.1002/adfm.202010747 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

