ZmPHR1 contributes to drought resistance by modulating phosphate homeostasis in maize
- PMID: 39037027
- PMCID: PMC11500998
- DOI: 10.1111/pbi.14431
ZmPHR1 contributes to drought resistance by modulating phosphate homeostasis in maize
Abstract
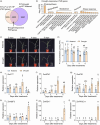
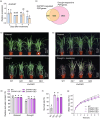
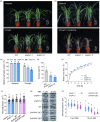
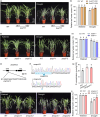
As an essential macronutrient, phosphorus (P) is often a limiting nutrient because of its low availability and mobility in soils. Drought is a major environmental stress that reduces crop yield. How plants balance and combine P-starvation responses (PSRs) and drought resistance is unclear. In this study, we identified the transcription factor ZmPHR1 as a major regulator of PSRs that modulates phosphate (Pi) signaling and homeostasis. We found that maize zmphr1 mutants had reduced P concentration and were sensitive to Pi starvation, whereas ZmPHR1-OE lines displayed elevated Pi concentration and yields. In addition, 57% of PSR genes and nearly 70% of ZmPHR1-regulated PSR genes in leaves were transcriptionally responsive to drought. Under moderate and early drought conditions, the Pi concentration of maize decreased, and PSR genes were up-regulated before drought-responsive genes. The ZmPHR1-OE lines exhibited drought-resistant phenotypes and reduced stomatal apertures, whereas the opposite was true of the zmphr1 mutants. ZmPT7-OE lines and zmspx3 mutants, which had elevated Pi concentration, also exhibited drought resistance, but zmpt7 mutants were sensitive to drought. Our results suggest that ZmPHR1 plays a central role in integrating Pi and drought signals and that Pi homeostasis improves the ability of maize to combat drought.
Keywords: ZmPHR1; drought; maize; phosphate homeostasis.
© 2024 The Author(s). Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Figures



References
-
- Amanullah , Asif, M. , Nawab, K. , Shah, Z. , Hassan, M. , Khan, A.Z. , Khalil, S.K. et al. (2010) Impact of planting density and P‐fertilizer source on the growth analysis of maize. Pak. J. Bot. 42, 2349–2357.
-
- Burman, U. , Garg, B.K. and Kathju, S. (2009) Effect of phosphorus application on clusterbean under different intensities of water stress. J. Plant Nutr. 32, 668–680.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

