Toll-like receptor 4 (TLR4) is the major pattern recognition receptor triggering the protective effect of a Candida albicans extracellular vesicle-based vaccine prototype in murine systemic candidiasis
- PMID: 39037263
- PMCID: PMC11351041
- DOI: 10.1128/msphere.00467-24
Toll-like receptor 4 (TLR4) is the major pattern recognition receptor triggering the protective effect of a Candida albicans extracellular vesicle-based vaccine prototype in murine systemic candidiasis
Abstract
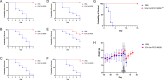
Systemic candidiasis remains a significant public health concern worldwide, with high mortality rates despite available antifungal drugs. Drug-resistant strains add to the urgency for alternative therapies. In this context, vaccination has reemerged as a prominent immune-based strategy. Extracellular vesicles (EVs), nanosized lipid bilayer particles, carry a diverse array of native fungal antigens, including proteins, nucleic acids, lipids, and glycans. Previous studies from our laboratory demonstrated that Candida albicans EVs triggered the innate immune response, activating bone marrow-derived dendritic cells (BMDCs) and potentially acting as a bridge between innate and adaptive immunity. Vaccination with C. albicans EVs induced the production of specific antibodies, modulated cytokine production, and provided protection in immunosuppressed mice infected with lethal C. albicans inoculum. To elucidate the mechanisms underlying EV-induced immune activation, our study investigated pathogen-associated molecular patterns (PAMPs) and pattern recognition receptors (PRRs) involved in EVs-phagocyte engagement. EVs from wild-type and mutant C. albicans strains with truncated mannoproteins were compared for their ability to stimulate BMDCs. Our findings revealed that EV decoration with O- and N-linked mannans and the presence of β-1,3-glucans and chitin oligomers may modulate the activation of specific PRRs, in particular Toll-like receptor 4 (TLR4) and dectin-1. The protective effect of vaccination with wild-type EVs was found to be dependent on TLR4. These results suggest that fungal EVs can be harnessed in vaccine formulations to selectively activate PRRs in phagocytes, offering potential avenues for combating or preventing candidiasis.IMPORTANCESystemic candidiasis is a serious global health concern with high mortality rates and growing drug resistance. Vaccination offers a promising solution. A unique approach involves using tiny lipid-coated particles called extracellular vesicles (EVs), which carry various fungal components. Previous studies found that Candida albicans EVs activate the immune response and may bridge the gap between innate and adaptive immunity. To understand this better, we investigated how these EVs activate immune cells. We demonstrated that specific components on EV surfaces, such as mannans and glucans, interact with receptors on immune cells, including Toll-like receptor 4 (TLR4) and dectin-1. Moreover, vaccinating with these EVs led to strong immune responses and full protection in mice infected with Candida. This work shows how harnessing fungal EVs might lead to effective vaccines against candidiasis.
Keywords: Candida albicans; extracellular vesicles; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


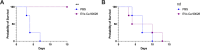
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 408843/2021-7, 405520/2018-2, 440015/2018-9, 311179/2017-7, 408711/2017-7, 408843/2021-7/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)
- MR/N006364/2/Medical Research Council Centre for Medical Mycology (MRC CMM)
- 26/211.300/2021, 26/202.696/2018, 26/202.809/2018, 26/201.032/2022/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ)
- MR/M026663/2/UKRI | Medical Research Council (MRC)
- National Institute for Health and Care Research (NIHR)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

