Flagellar stator genes control a trophic shift from obligate to facultative predation and biofilm formation in a bacterial predator
- PMID: 39037271
- PMCID: PMC11323537
- DOI: 10.1128/mbio.00715-24
Flagellar stator genes control a trophic shift from obligate to facultative predation and biofilm formation in a bacterial predator
Abstract
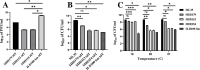
The bacterial predator Bdellovibrio bacteriovorus is considered to be obligatorily prey (host)-dependent (H-D), and thus unable to form biofilms. However, spontaneous host-independent (H-I) variants grow axenically and can form robust biofilms. A screen of 350 H-I mutants revealed that single mutations in stator genes fliL or motA were sufficient to generate flagellar motility-defective H-I strains able to adhere to surfaces but unable to develop biofilms. The variants showed large transcriptional shifts in genes related to flagella, prey-invasion, and cyclic-di-GMP (CdG), as well as large changes in CdG cellular concentration relative to the H-D parent. The introduction of the parental fliL allele resulted in a full reversion to the H-D phenotype, but we propose that specific interactions between stator proteins prevented functional complementation by fliL paralogs. In contrast, specific mutations in a pilus-associated protein (Bd0108) mutant background were necessary for biofilm formation, including secretion of extracellular DNA (eDNA), proteins, and polysaccharides matrix components. Remarkably, fliL disruption strongly reduced biofilm development. All H-I variants grew similarly without prey, showed a strain-specific reduction in predatory ability in prey suspensions, but maintained similar high efficiency in prey biofilms. Population-wide allele sequencing suggested additional routes to host independence. Thus, stator and invasion pole-dependent signaling control the H-D and the H-I biofilm-forming phenotypes, with single mutations overriding prey requirements, and enabling shifts from obligate to facultative predation, with potential consequences on community dynamics. Our findings on the facility and variety of changes leading to facultative predation also challenge the concept of Bdellovibrio and like organisms being obligate predators.
Importance: The ability of bacteria to form biofilms is a central research theme in biology, medicine, and the environment. We show that cultures of the obligate (host-dependent) "solitary" predatory bacterium Bdellovibrio bacteriovorus, which cannot replicate without prey, can use various genetic routes to spontaneously yield host-independent (H-I) variants that grow axenically (as a single species, in the absence of prey) and exhibit various surface attachment phenotypes, including biofilm formation. These routes include single mutations in flagellar stator genes that affect biofilm formation, provoke motor instability and large motility defects, and disrupt cyclic-di-GMP intracellular signaling. H-I strains also exhibit reduced predatory efficiency in suspension but high efficiency in prey biofilms. These changes override the requirements for prey, enabling a shift from obligate to facultative predation, with potential consequences on community dynamics.
Keywords: Bdellovibrios; cyclic di-GMP; flagellar motility; predation; stator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


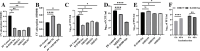



References
-
- Cohen Y, Pasternak Z, Müller S, Hübschmann T, Schattenberg F, Sivakala KK, Abed-Rabbo A, Chatzinotas A, Jurkevitch E. 2021. Community and single cell analyses reveal complex predatory interactions between bacteria in high diversity systems. Nat Commun 12:5481. doi:10.1038/s41467-021-25824-9 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
