Chimeric lipoproteins for leptospirosis vaccine: immunogenicity and protective potential
- PMID: 39037584
- PMCID: PMC11263434
- DOI: 10.1007/s00253-024-13196-1
Chimeric lipoproteins for leptospirosis vaccine: immunogenicity and protective potential
Abstract
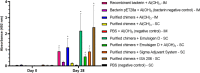
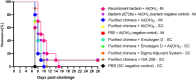
Leptospirosis, a neglected zoonotic disease, is caused by pathogenic spirochetes belonging to the genus Leptospira and has one of the highest morbidity and mortality rates worldwide. Vaccination stands out as one of the most effective preventive measures for susceptible populations. Within the outer membrane of Leptospira spp., we find the LIC12287, LIC11711, and LIC13259 lipoproteins. These are of interest due to their surface location and potential immunogenicity. Thorough examination revealed the conservation of these proteins among pathogenic Leptospira spp.; we mapped the distribution of T- and B-cell epitopes along their sequences and assessed the 3D structures of each protein. This information aided in selecting immunodominant regions for the development of a chimeric protein. Through gene synthesis, we successfully constructed a chimeric protein, which was subsequently expressed, purified, and characterized. Hamsters were immunized with the chimeric lipoprotein, formulated with adjuvants aluminum hydroxide, EMULSIGEN®-D, Sigma Adjuvant System®, and Montanide™ ISA206VG. Another group was vaccinated with an inactivated Escherichia coli bacterin expressing the chimeric protein. Following vaccination, hamsters were challenged with a virulent L. interrogans strain. Our evaluation of the humoral immune response revealed the production of IgG antibodies, detectable 28 days after the second dose, in contrast to pre-immune samples and control groups. This demonstrates the potential of the chimeric protein to elicit a robust humoral immune response; however, no protection against challenge was achieved. While this study provides valuable insights into the subject, further research is warranted to identify protective antigens that could be utilized in the development of a leptospirosis vaccine. KEY POINTS: • Several T- and B-cell epitopes were identified in all the three proteins. • Four different adjuvants were used in vaccine formulations. • Immunization stimulated significant levels of IgG2/3 in vaccinated animals.
Keywords: Leptospira; Adjuvants; Chimeric protein; Recombinant bacterin; Recombinant inactivated E. coli vaccine; Recombinant subunit vaccine.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Chimeric epitope vaccine against Leptospira interrogans infection and induced specific immunity in guinea pigs.BMC Microbiol. 2016 Oct 14;16(1):241. doi: 10.1186/s12866-016-0852-y. BMC Microbiol. 2016. PMID: 27737644 Free PMC article.
-
Protective Immunity and Reduced Renal Colonization Induced by Vaccines Containing Recombinant Leptospira interrogans Outer Membrane Proteins and Flagellin Adjuvant.Clin Vaccine Immunol. 2015 Aug;22(8):965-73. doi: 10.1128/CVI.00285-15. Epub 2015 Jun 24. Clin Vaccine Immunol. 2015. PMID: 26108285 Free PMC article.
-
Design and evaluation of potent multiepitope broad spectrum DNA and protein vaccine candidates against leptospirosis.Microb Pathog. 2025 May;202:107418. doi: 10.1016/j.micpath.2025.107418. Epub 2025 Feb 27. Microb Pathog. 2025. PMID: 40023457
-
Vaccines against leptospirosis.Curr Top Microbiol Immunol. 2015;387:251-72. doi: 10.1007/978-3-662-45059-8_10. Curr Top Microbiol Immunol. 2015. PMID: 25388138 Review.
-
Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen.Nat Rev Microbiol. 2009 Oct;7(10):736-47. doi: 10.1038/nrmicro2208. Nat Rev Microbiol. 2009. PMID: 19756012 Free PMC article. Review.
References
-
- Adler B (2015) Vaccines against leptospirosis. Curr Top Microbiol Immunol 387:251–272. 10.1007/978-3-662-45059-8_10 - PubMed
-
- Adler B, de la Peña Moctezuma A (2010) Leptospira and leptospirosis. Vet Microbiol 140:287–296. 10.1016/j.vetmic.2009.03.012 - PubMed
-
- Almagro Armenteros JJ, Tsirigos KD, Sønderby CK, Petersen TN, Winther O, Brunak S, von Heijne G, Nielsen H (2019) SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol 37:420–423. 10.1038/s41587-019-0036-z - PubMed
-
- Balakrishnan G, Roy P (2014) Comparision of efficacy of two experimental bovine Leptospira vaccines under laboratory and field. Vet Immunol Immunopathol 159:11–15. 10.1016/j.vetimm.2014.03.002 - PubMed
-
- Bettin EB, Dorneles J, Hecktheuer AS, Madruga AB, Seixas Neto ACP, McBride AJA, Oliveira TL, Grassmann AA, Dellagostin OA (2022) TonB-dependent receptor epitopes expressed in M. bovis BCG induced significant protection in the hamster model of leptospirosis. Appl Microbiol Biotechnol 106:173–184. 10.1007/s00253-021-11726-9 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

