APOER2 splicing repertoire in Alzheimer's disease: Insights from long-read RNA sequencing
- PMID: 39038048
- PMCID: PMC11293713
- DOI: 10.1371/journal.pgen.1011348
APOER2 splicing repertoire in Alzheimer's disease: Insights from long-read RNA sequencing
Abstract
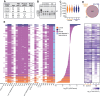
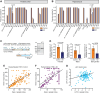
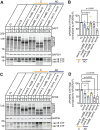
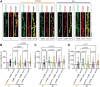
Disrupted alternative splicing plays a determinative role in neurological diseases, either as a direct cause or as a driver in disease susceptibility. Transcriptomic profiling of aged human postmortem brain samples has uncovered hundreds of aberrant mRNA splicing events in Alzheimer's disease (AD) brains, associating dysregulated RNA splicing with disease. We previously identified a complex array of alternative splicing combinations across apolipoprotein E receptor 2 (APOER2), a transmembrane receptor that interacts with both the neuroprotective ligand Reelin and the AD-associated risk factor, APOE. Many of the human APOER2 isoforms, predominantly featuring cassette splicing events within functionally important domains, are critical for the receptor's function and ligand interaction. However, a comprehensive repertoire and the functional implications of APOER2 isoforms under both physiological and AD conditions are not fully understood. Here, we present an in-depth analysis of the splicing landscape of human APOER2 isoforms in normal and AD states. Using single-molecule, long-read sequencing, we profiled the entire APOER2 transcript from the parietal cortex and hippocampus of Braak stage IV AD brain tissues along with age-matched controls and investigated several functional properties of APOER2 isoforms. Our findings reveal diverse patterns of cassette exon skipping for APOER2 isoforms, with some showing region-specific expression and others unique to AD-affected brains. Notably, exon 15 of APOER2, which encodes the glycosylation domain, showed less inclusion in AD compared to control in the parietal cortex of females with an APOE ɛ3/ɛ3 genotype. Also, some of these APOER2 isoforms demonstrated changes in cell surface expression, APOE-mediated receptor processing, and synaptic number. These variations are likely critical in inducing synaptic alterations and may contribute to the neuronal dysfunction underlying AD pathogenesis.
Copyright: © 2024 Gallo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

