Highly restrictive and directional penetration of the blood cerebral spinal fluid barrier by JCPyV
- PMID: 39038049
- PMCID: PMC11293668
- DOI: 10.1371/journal.ppat.1012335
Highly restrictive and directional penetration of the blood cerebral spinal fluid barrier by JCPyV
Abstract
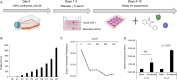
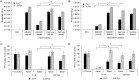
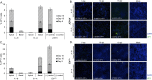
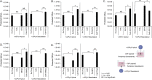
The human polyomavirus JCPyV is an opportunistic pathogen that infects greater than 60% of the world's population. The virus establishes a persistent and asymptomatic infection in the urogenital system but can cause a fatal demyelinating disease in immunosuppressed or immunomodulated patients following invasion of the CNS. The mechanisms responsible for JCPyV invasion into CNS tissues are not known but direct invasion from the blood to the cerebral spinal fluid via the choroid plexus has been hypothesized. To study the potential of the choroid plexus as a site of neuroinvasion, we used an adult human choroid plexus epithelial cell line to model the blood-cerebrospinal fluid (B-CSF) barrier in a transwell system. We found that these cells formed a highly restrictive barrier to virus penetration either as free virus or as virus associated with extracellular vesicles (EVJC+). The restriction was not absolute and small amounts of virus or EVJC+ penetrated and were able to establish foci of infection in primary astrocytes. Disruption of the barrier with capsaicin did not increase virus or EVJC+ penetration leading us to hypothesize that virus and EVJC+ were highly cell-associated and crossed the barrier by an active process. An inhibitor of macropinocytosis increased virus penetration from the basolateral (blood side) to the apical side (CSF side). In contrast, inhibitors of clathrin and raft dependent transcytosis reduced virus transport from the basolateral to the apical side of the barrier. None of the drugs inhibited apical to basolateral transport suggesting directionality. Pretreatment with cyclosporin A, an inhibitor of P-gp, MRP2 and BCRP multidrug resistance transporters, restored viral penetration in cells treated with raft and clathrin dependent transcytosis inhibitors. Because choroid plexus epithelial cells are known to be susceptible to JCPyV infection both in vitro and in vivo we also examined the release of infectious virus from the barrier. We found that virus was preferentially released from the cells into the apical (CSF) chamber. These data show clearly that there are two mechanisms of penetration, direct transcytosis which is capable of seeding the CSF with small amounts of virus, and infection followed by directional release of infectious virions into the CSF compartment.
Copyright: © 2024 O’Hara et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Cortese I, Beck ES, Al-Louzi O, Ohayon J, Andrada F, Osuorah I, et al.. BK virus-specific T cells for immunotherapy of progressive multifocal leukoencephalopathy: an open-label, single-cohort pilot study. Lancet Neurol. 2021;20(8):639–52. Epub 2021/07/25. doi: 10.1016/S1474-4422(21)00174-5 ; PubMed Central PMCID: PMC8395368. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

