Identifying and Engineering Flavin Dependent Halogenases for Selective Biocatalysis
- PMID: 39038085
- PMCID: PMC11309780
- DOI: 10.1021/acs.accounts.4c00172
Identifying and Engineering Flavin Dependent Halogenases for Selective Biocatalysis
Abstract
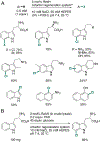
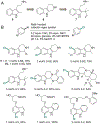
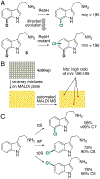
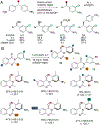
Organohalogen compounds are extensively used as building blocks, intermediates, pharmaceuticals, and agrochemicals due to their unique chemical and biological properties. Installing halogen substituents, however, frequently requires functionalized starting materials and multistep functional group interconversion. Several classes of halogenases evolved in nature to enable halogenation of a different classes of substrates; for example, site-selective halogenation of electron rich aromatic compounds is catalyzed by flavin-dependent halogenases (FDHs). Mechanistic studies have shown that these enzymes use FADH2 to reduce O2 to water with concomitant oxidation of X- to HOX (X = Cl, Br, I). This species travels through a tunnel within the enzyme to access the FDH active site. Here, it is believed to interact with an active site lysine proximal to bound substrate, enabling electrophilic halogenation with selectivity imparted via molecular recognition, rather than directing groups or strong electronic activation.The unique selectivity of FDHs led to several early biocatalysis efforts, preparative halogenation was rare, and the hallmark catalyst-controlled selectivity of FDHs did not translate to non-native substrates. FDH engineering was limited to site-directed mutagenesis, which resulted in modest changes in site-selectivity or substrate preference. To address these limitations, we optimized expression conditions for the FDH RebH and its cognate flavin reductase (FRed), RebF. We then showed that RebH could be used for preparative halogenation of non-native substrates with catalyst-controlled selectivity. We reported the first examples in which the stability, substrate scope, and site selectivity of a FDH were improved to synthetically useful levels via directed evolution. X-ray crystal structures of evolved FDHs and reversion mutations showed that random mutations throughout the RebH structure were critical to achieving high levels of activity and selectivity on diverse aromatic substrates, and these data were used in combination with molecular dynamics simulations to develop predictive model for FDH selectivity. Finally, we used family wide genome mining to identify a diverse set of FDHs with novel substrate scope and complementary regioselectivity on large, three-dimensionally complex compounds.The diversity of our evolved and mined FDHs allowed us to pursue synthetic applications beyond simple aromatic halogenation. For example, we established that FDHs catalyze enantioselective reactions involving desymmetrization, atroposelective halogenation, and halocyclization. These results highlight the ability of FDH active sites to tolerate different substrate topologies. This utility was further expanded by our recent studies on the single component FDH/FRed, AetF. While we were initially drawn to AetF because it does not require a separate FRed, we found that it halogenates substrates that are not halogenated efficiently or at all by other FDHs and provides high enantioselectivity for reactions that could only be achieved using RebH variants after extensive mutagenesis. Perhaps most notably, AetF catalyzes site-selective aromatic iodination and enantioselective iodoetherification. Together, these studies highlight the origins of FDH engineering, the utility and limitations of the enzymes developed to date, and the promise of FDHs for an ever-expanding range of biocatalytic halogenation reactions.
Conflict of interest statement
The author declares no competing financial interest.
Figures







References
-
- Fisher BF; Snodgrass HM; Jones KA; Andorfer MC; Lewis JC Site-Selective C–H Halogenation Using Flavin-Dependent Halogenases Identified via Family-Wide Activity Profiling. ACS Cent. Sci 2019, 5 (11), 1844–1856. - PMC - PubMed
-
Genome mining was used to identify over 50 new functional FDHs that enable site-selective halogenation of diverse small molecules.
-
- Mondal D; Fisher BF; Jiang Y; Lewis JC Flavin-Dependent Halogenases Catalyze Enantioselective Olefin Halocyclization. Nat. Commun 2021, 12 (1), 3268. - PMC - PubMed
-
The first report of a FDH catalyzing a non-native transformation, and the first enantioselective FDH catalysis involving olefin halogenation.
-
- Jiang Y; Snodgrass HM; Zubi YS; Roof CV; Guan Y; Mondal D; Honeycutt NH; Lee JW; Lewis RD; Martinez CA; Lewis JC The Single-Component Flavin Reductase/Flavin-Dependent Halogenase AetF Is a Versatile Catalyst for Selective Bromination and Iodination of Arenes and Olefins**. Angew. Chem., Int. Ed 2022, 61 (51), No. e202214610. - PMC - PubMed
-
The first report of a single component FDH catalyzing non-native transformations with catalyst controlled site- and enantioselectivity.
-
- Hedstrom L Serine Protease Mechanism and Specificity. Chem. Rev 2002, 102 (12), 4501–4524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

